Endobronchial ultrasound-transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: variability of results and perspectives
Introduction
Over the last 15 years endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) has emerged as the technique of first choice for biopsy of mediastinal lymph nodes (LN). In patients with suspect/known non-small cell lung cancer (NSCLC), the primary indications of EBUS-TBNA are tissue confirmation of computed tomography (CT)-enlarged or positron emission tomography (PET)-positive mediastinal LN, and systematic mediastinal LN staging, a major determinant of tumor resectability and prognosis. In selected patients with suspect lung cancer the EBUS-TBNA technique may be used as first-line tissue sampling procedure; if this shows N2/N3 invasion and identifies the lung cancer subtype, simultaneous diagnosis and mediastinal staging of the disease can be obtained, and the duration and cost of the diagnostic process are reduced. Moreover, it may be helpful averting lung tumor biopsy when this is technically difficult, or high-risk (e.g., emphysematous patients), or inappropriate (e.g., patient unfit for surgery).
EBUS-TBNA for diagnosis of malignancy in mediastinal nodes is a highly effective technique, with a low (about 1%) rate of complications, almost exclusively minor (1-5). In the context of NSCLC diagnosis and staging, reports from different centers show considerable variation of EBUS-TBNA diagnostic yield (71–99%) (5-9), and of key performance indices, namely sensitivity (46–97%) and negative predictive value (NPV) (60–99%) (10). Discrepancies in the reported efficacy of the procedure and lack of generally accepted benchmarks for EBUS-TBNA outcomes complicate the comparison of findings across institutions. The performance indices of EBUS-TNBA mediastinal staging may be influenced by numerous factors (Table 1), the most relevant of which are the N2/N3 disease prevalence and the thoroughness of staging (10-12). We will focus on these factors, as they are important for interpreting differences in the procedure efficacy among institutions and for establishing quality standards of EBUS-TBNA practice.
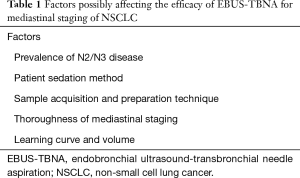
Full table
Prevalence of N2/N3 disease
Prevalence of N2/N3 disease in the tested population of NSCLC patients impacts on the diagnostic performance of EBUS-TBNA (12-14). As indicated by the American College of Chest Physicians (ACCP) meta-analysis of EBUS-TBNA mediastinal staging, which included a total of 2,756 procedures (10), the prevalence of N2/N3 disease directly correlates with diagnostic sensitivity and inversely correlates with NPV: with <20% prevalence of mediastinal lymph nodal metastases, EBUS-TBNA showed 78% sensitivity and 96% NPV; with ≥80% prevalence, 96% sensitivity and 83% NPV (12). Because prevalence of mediastinal nodal metastasis is a major bias, it is not surprising that EBUS-TBNA diagnostic performance varies among series with different N2/N3 disease prevalence, and staging results are difficult to translate from one study to another (15). For meaningful assessment of the quality of EBUS-TBNA outcomes, it is necessary to know the prevalence of mediastinal nodal metastasis in the examined population (12).
Patient sedation method
Patient sedation for conducting EBUS-TBNA can be obtained by conscious sedation (usually with fentanyl and midazolam) or by general anesthesia (with propofol and oro-tracheal tube/laryngeal mask). Proponents of general anesthesia maintain that abolishing respiratory variation and cough facilitates sampling when target nodes are small and the patient is scarcely collaborating. Whether the type of sedation makes a difference in the procedure performance has been debated. There are no randomized studies documenting a superior diagnostic performance of EBUS-TBNA with either sedation method. In a retrospective multicentric study of EBUS-TBNA that we recently carried out, sampling adequacy rate was similar with general anesthesia and with conscious sedation (87% and 92%; P=0.09) (16), in agreement with the conclusion of current systematic literature reviews indicating that the choice of sedation method can be left to the operator preference (17,18).
Sample acquisition and preparation techniques
EBUS-TBNA procedures are generally performed with 21-Gauge (G) or 22-G needle. After reviewing the evidence from studies comparing the diagnostic yield of EBUS-TBNA with these two needle sizes, Wahidi et al. concluded that either size is an acceptable option (18). Some authors prefer the larger-bore needle (21-G) because it provides more tissue material, which can be used for histology and for molecular studies. In the context of potentially operable NSCLC, for thorough assessment of mediastinal node involvement it is generally agreed that 3 needle passes per target LN should be obtained, if rapid on-site evaluation (ROSE) of samples is not used (17-20). Each pass should include 5 to 15 needle agitations within the target node (18), with or without suction (21). The important indication that three is the optimal number of punctures per target LN was provided by the study of Lee et al. who evaluated by EBUS-TBNA 163 LN stations in 102 NSCLC patients (19). In that study each target LN was punctured four times, however after three passes the sample adequacy was 100%; the sensitivity for differentiating malignant from benign LN stations was 95.3% and did not increase with four passes (19).
The most frequently used techniques for EBUS-TBNA specimen acquisition and processing are cytology slides, cell-block, core-tissue, combination of cytology slides and core-tissue, combination of cytology slides and cell-block. Only few studies comparing these techniques have been published and there is no consensus on the optimal method of specimen preparation (17). In our institute a study was carried out to identify the best performing technique among those currently available for EBUS-TBNA specimen acquisition and processing; we found that the diagnostic yield with cytology smear and with core-tissue were high and similar (81% vs. 87%; P=0.44) (22). In the literature, like in our study, the comparison of cytology smear, core-tissue and cell-block methods showed that no single method is superior to the others (23,24). Notably, we attained the highest diagnostic yield (100%) by combining two methods (cytology slides and core-tissue, or cytology slides and cell-block); however the use of two methods in combination is expensive and time-consuming (22). Considering that familiarity and expertise with a technique impacts on the final results, choice of needle size to use for EBUS-TBNA and of specimen processing method should be left to the operator experience and preference (17).
The influence of ROSE on quantity, quality and yield of EBUS-TBNA samples in patients with known or suspect lung cancer has been the object of a systematic literature review by Van der Heijden et al. (17). These authors provided guidelines for specimen acquisition and preparation, indicating that ROSE does not modify EBUS-TBNA diagnostic yield, nor does it affect the number of needle passes, the duration of the procedure, and the complication rate. However, when EBUS-TBNA was the first diagnostic procedure in patients with suspect lung cancer, ROSE was found to reduce the number of additional procedures (25,26).
In conclusion, the sample acquisition and preparation technique are unlikely to impact on EBUS-TBNA diagnostic yield, provided that at least three needle passes per target LN are done (in the absence of ROSE of samples).
Thoroughness of mediastinal staging
Mediastinal nodal staging is a critical step for determining the best treatment of NSCLC. For this purpose, until recently mediastinoscopy was the generally accepted gold standard, with about 80% sensitivity and about 90% NPV in confirming N2/N3 disease (10). Pretreatment mediastinal staging of NSCLC has been revolutioned by the advent of EBUS-TBNA, a procedure characterized by sensitivity equivalent to that of mediastinoscopy (Table 2), but less invasive, less risky, and obviating the need for general anesthesia (10). As a results, in recent years EBUS-TBNA has almost replaced surgical staging by mediastinoscopy in many centers (6,12).
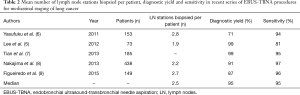
Full table
It has been remarked that the reliability of mediastinal staging with EBUS-TBNA, as with other staging techniques, largely depends on the thoroughness of the procedure (10,11,13,19). Thorough mediastinal staging by mediastinoscopy or EBUS-TBNA is best performed with systematic sampling, which requires biopsy of representative LNs in stations 2R, 2L, 4R, 4L, 7 (10). Current guidelines recommend aiming to target at least 3 LN stations (typically 4R, 4L and 7), including those LNs with CT/PET features suggestive of metastasis (6,10,12,20).
Despite these recommendations, in real life the EBUS-TBNA practice is generally characterized by <3 sampled mediastinal nodal stations per patient (27,28), for multiple reasons that include difficult punture of LNs, bleeding, restless patient, longer procedure, ROSE showing metastasis early in the procedure. In early reports of EBUS-TBNA the median number of mediastinal LN stations sampled per patient was <2 (5), a result that has improved in more recent series (Table 2) and that needs further improvement (12). Limited thoroughness of mediastinal staging likely contributes to the variability of EBUS-TBNA staging accuracy in different studies.
Whether mediastinoscopy should be routinely done after negative EBUS-TBNA remains controversial (6,29-31). The prospective randomized trial of EBUS-TBNA vs. mediastinoscopy for staging of NSCLC performed by Yasufuku et al. showed equivalent effectiveness of the two techniques in determining the true pathologic N stage (6). However, for EBUS-TBNA negative cases at high risk of lung cancer metastases, the current ACCP and British Thoracic Society (BTS) guidelines recommend mediastinoscopy or other surgical approaches to obtain mediastinal nodes’ biopsy (10,32).
Learning curve and volume
The skill and experience of the operator, usually an interventional pulmonologist or a thoracic surgeon, influence EBUS-TBNA results. For acquisition of the EBUS-TBNA technique the ACCP, the European Respiratory Society and the American Thoracic Society recommend an initial training of 40–50 supervised procedures. In addition, 20 procedures per year are suggested for maintaining competency (33,34). The speed of learning the EBUS-TBNA procedure varies for different operators, either highly experienced or trainee bronchoscopist (35,36). Furthermore, improvements in performance are reported even after doing 200 procedures, indicating that volume affects the quality of EBUS-TBNA outcomes (36,37). In a recent EBUS-TBNA workshop held in October 2016 in Varese, Italy, a multicentric study was presented that included 485 EBUS-TBNA mediastinal staging procedures in NSCLC patients and compared the outcomes of the five participating units. In all those centers the prevalence of N2 disease was >70%, but wide variation was found in the rate of inadequate sampling (0–29%) and in the rate of false-negative (FN) EBUS-TBNA results (4–11% of all adequate samples); interestingly, the center with the largest EBUS volume (50 procedures/year) showed the lowest FN rate (4%), and the FN rate inversely correlated (P<0.009) with the volume of procedures in the individual centers (38).
To date, the length of the learning curve for EBUS-TBNA proficiency is unclear and is probably different for each operator; no diagnostic yield cut-off has yet been established to define the standard capability of performing EBUS-TBNA.
Perspectives
Molecular testing
Improvement of target therapy makes tumor subtyping and genotyping increasingly necessary for management of lung cancer; obtaining from EBUS-TBNA a specimen suitable for molecular analysis is therefore important. The success in performing molecular tests on TBNA samples depends on the absolute number and percentage of malignant cells present in the sampled material, on quality of cell preservation and on type and sensitivity of the test itself (17,39). Recent reports from high-volume centers indicate that 72–97% of EBUS-TBNA samples are appropriate for testing the most frequently used prognostic markers of lung cancer, namely EGFR, ALK and KRAS (17,25,39,40). Both smear and cell block preparation, or core tissue, can be utilized for molecular testing (17,23); however, while EGFR and KRAS status can be determined using all three specimen preparation techniques, the ALK translocation is best assessed using cell block and core tissue (17). Cell block and core tissue are currently judged to be the best material for molecular analysis, suggesting to privilege these two processing methods if possible.
In advanced lung cancer patients whose treatment may vary on the molecular results, a recent randomized trial showed that ROSE increased by 10% (although not significantly) the success rate of EBUS-TBNA for genotyping, and reduced the number of redo procedures and of further investigations (25).
No randomized studies are available on the influence of needle size, use of suction and type of sedation on the adequacy of samples for molecular testing. In patients undergoing EBUS-TBNA for diagnosis and/or staging of suspect/known NSCLC, more than three needle passes may be necessary to obtain sufficient material for molecular testing (18,30); it is therefore recommended that additional samples be obtained for EGFR and ALK testing (41).
Quality standards
The vast majority of published EBUS-TBNA studies consists of retrospective cohort reports without pre-defined standards and represents real life practice. Efforts should be made to shift from targeted sampling of enlarged or PET positive nodes to systematic mediastinal node sampling (12); this will likely improve the thoroughness of staging, the accuracy of EBUS-TBNA and the interpretation of local performance data. EBUS-TBNA guidelines have been made available from expert centers (17,18) but quality standards for assessment of EBUS-TBNA performance have not yet been established, with the exception of the 2014 BTS Quality Standard that states a minimum of 88% sensitivity for mediastinal staging of suspected NSCLC (42). EBUS-TBNA practice needs to be standardized to ensure the best outcomes in all institutions (12). To this effect the performance of staging could be measured by the sensitivity and NPV metrics, stratifying the population by prevalence of N2/N3 disease, because the latter influences both sensitivity and NPV. The ACCP guidelines on NSCLC staging classify patients into four groups (A, B, C, D), according to the index staging CT scan of the chest (10); this classification correlates with N2/N3 disease prevalence and may be used to define standards for nodal staging and for comparison of EBUS-TBNA outcomes across centers (12). Importantly, for meaningful comparison of EBUS-TBNA results, the data should be gathered prospectively, because those retrospectively collected are frequently uncertain as to the indication to perform EBUS-TBNA (diagnosis vs. mediastinal staging), the prevalence of N2/N3 disease, and the exact LN stations successfully sampled. An important step forward will be made when the use of a standardized database for prospective collection of relevant EBUS-TBNA data will be generalized (12).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest 2013;143:1044-53. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J 2009;33:1156-64. [Crossref] [PubMed]
- Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest 2007;131:539-48. [Crossref] [PubMed]
- Lee BE, Kletsman E, Rutledge JR, et al. Utility of endobronchial ultrasound-guided mediastinal lymph node biopsy in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2012;143:585-90. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400. [Crossref] [PubMed]
- Tian Q, Chen L, Wang R, et al. The reason of false negative results of endobronchial ultrasound-transbronchial needle aspiration in the diagnosis of intrapulmonary and mediastinal malignancy. Thoracic Cancer 2013;4:186-90. [Crossref]
- Nakajima T, Yasufuku K, Saegusa F, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for nodal staging in patients with lung cancer. Ann Thorac Surg 2013;95:1695-9. [Crossref] [PubMed]
- Figueiredo VR, Cardoso PF, Jacomelli M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for lung cancer staging: early experience in Brazil. J Bras Pneumol 2015;41:23-30. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-20S.
- Evison M, Crosbie P, Navani N, et al. How should performance in EBUS mediastinal staging in lung cancer be measured? Br J Cancer 2016;115:e9. [Crossref] [PubMed]
- Kennedy MP, Jimenez CA, Morice RC, et al. Factors influencing the diagnostic Yield of Endobronchial Ultrasound-guided Transbronchial Needle Aspiration. J Bronchology Interv Pulmonol 2010;17:202-8. [Crossref] [PubMed]
- Holty JE, Kuschner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax 2005;60:949-55. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Eloubeidi MA, et al. The true false negative rates of esophageal and endobronchial ultrasound in the staging of mediastinal lymph nodes in patients with non-small cell lung cancer. Ann Thorac Surg 2010;90:427-34. [Crossref] [PubMed]
- Rotolo N, Imperatori A, Nosotti M, et al. Multicentric study of endobronchial ultrasound-transbronchial needle aspiration for lung cancer staging in Italy. J Thorac Dis 2017;9:S370-S375.
- Van der Heijden EH, Casal RF, Trisolini R, et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014;88:500-17. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest 2008;134:368-74. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Casal RF, Staerkel GA, Ost D, et al. Randomized clinical trial of endobronchial ultrasound needle biopsy with and without aspiration. Chest 2012;142:568-73. [Crossref] [PubMed]
- Rotolo N, Cattoni M, Crosta G, et al. Comparison of multiple techniques for endobronchial ultrasound-transbronchial needle aspiration specimen preparation in a single institution experience. J Thorac Dis 2017;9:S381-S385.
- Navani N, Brown JM, Nankivell M, et al. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: a multicenter study of 774 patients. Am J Respir Crit Care Med 2012;185:1316-22. [Crossref] [PubMed]
- Steinfort DP, Russell PA, Tsui A, et al. Interobserver agreement in determining non-small cell lung cancer subtype in specimens acquired by EBUS- TBNA. Eur Respir J 2012;40:699-705. [Crossref] [PubMed]
- Trisolini R, Cancellieri A, Tinelli C, et al. Randomized Trial of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration With and Without Rapid On-site Evaluation for Lung Cancer Genotyping. Chest 2015;148:1430-7. [Crossref] [PubMed]
- Oki M, Saka H, Ando M, et al. Endoscopic ultrasound-guided fine needle aspiration and endobronchial ultrasound-guided transbronchial needle aspiration: Are two better than one in mediastinal staging of non-small cell lung cancer? J Thorac Cardiovasc Surg 2014;148:1169-77. [Crossref] [PubMed]
- Jeebun V, Harrison RN. Understanding local performance data for EBUS-TBNA: insights from an unselected case series at a high volume UK center. J Thorac Dis 2017. [Epub ahead of print].
- Evison M, Crosbie P, Martin J, et al. EBUS-guided mediastinal lung cancer staging: monitoring of quality standards improves performance. Thorax 2016;71:762-3. [Crossref] [PubMed]
- Taverner J, Cheang MY, Antippa P. Negative EBUS-TBNA predicts very low prevalence of mediastinal disease in staging of non-small cell lung cancer. J Bronchology Interv Pulmonol 2016;23:177-80. [Crossref] [PubMed]
- Kinsey CM, Arenberg DA. Endobronchial ultrasound-guided transbronchial needle aspiration for non-small cell lung cancer staging. Am J Respir Crit Care Med 2014;189:640-9. [Crossref] [PubMed]
- Rusch VW. Mediastinoscopy: an obsolete procedure? J Thorac Cardiovasc Surg 2011;142:1400-2. [Crossref] [PubMed]
- Du Rand IA, Barber PV, Goldring J, et al. Summary of the British Thoracic Society guidelines for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax 2011;66:1014-5. [Crossref] [PubMed]
- Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest 2003;123:1693-717. [Crossref] [PubMed]
- Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J 2002;19:356-73. [PubMed]
- Kemp SV, El Batrawy SH, Harrison RN, et al. Learning curves for endobronchial ultrasound using cusum analysis. Thorax 2010;65:534-8. [Crossref] [PubMed]
- Stather DR, Chee A, MacEachern P, et al. Endobronchial ultrasound learning curve in interventional pulmonary fellows. Respirology 2015;20:333-9. [Crossref] [PubMed]
- Hu Y, Puri V, Crabtree TD, et al. Attaining proficiency with endobronchial ultrasound-guided transbronchial needle aspiration. J Thorac Cardiovasc Surg 2013;146:1387-92. [Crossref] [PubMed]
- Rotolo N, Nosotti M, Santambrogio L, et al. False-negative rate and volume of EBUS-TBNA procedures for NSCLC staging: a multicenter study in Italy. Proceedings of the 25th European Conference on General Thoracic Surgery, Innsbruck, Austria, 2017.
- VanderLaan PA, Wang HH, Majid A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA): an overview and update for the cytopathologist. Cancer Cytopathol 2014;122:561-76. [Crossref] [PubMed]
- Casadio C, Guarize J, Donghi S, et al. Molecular Testing for Targeted Therapy in Advanced Non-Small Cell Lung Cancer: Suitability of Endobronchial Ultrasound Transbronchial Needle Aspiration. Am J Clin Pathol 2015;144:629-34. [Crossref] [PubMed]
- Yarmus L, Akulian J, Gilbert C, et al. Optimizing endobronchial ultrasound for molecular analysis. How many passes are needed? Ann Am Thorac Soc 2013;10:636-43. [Crossref] [PubMed]
- BTS Quality Standards for Flexible Bronchoscopy in Adults. Available online: https://www.brit-thoracic.org.uk/document-library/clinical-information/bronchoscopy/bts-quality-standards-for-flexible-bronchoscopy-2014/


