Remedial localization after dislodgement of primary mechanical localization in lung surgery
Introduction
Accurate localization of solitary pulmonary nodules (SPNs) has remained a big challenge in lung surgery, especially when the diameter is <15 mm and the nodule is >10 mm under the surface of visceral pleura (1). Computed tomography (CT)-guided percutaneous localization with hookwire or microcoil is one of the most commonly used localization techniques (2). Both hookwire and microcoil have been found to have high success rate and low complication rate in previous studies (3-5). Microcoil placement can also reduce conversion to thoracotomy, operation time, and stapler firings (6). However, unhooking and displacement, among other complications, like pneumothorax, hemorrhage, or dislodgement, do occur (5-8).
In reality, unhooking or displacement of hookwire or microcoil due to technical failures is rather common. Hookwire localization dislodgement rate varied between 3% and 8% in different studies (9-11). The dislodgement rate of microcoil localization, although less common, still reached 1.6% in a previous study (12). Unexpected displacement is of great concern because it may lead to decision dilemma during operations. One option is to close the chest and perform CT-guided localization again. It is time-consuming and rarely done in clinical practice. The other option is to perform tentative extended resections. However, this might lead to loss of more lung function and worsen the patient’s long-term prognosis (13,14). Thus, it is of great importance to find a practical method to avoid conversion to extended resection.
The remedial localization after dislodgement of primary mechanical localization in lung surgery is still to be studied. In this article, we hope to demonstrate a remedial technique for localizing small nodules in the case of dislodgement of primary localization. This study also evaluated the related effectiveness, efficacy, and safety of this new method.
Methods
Patients and primary localization
Informed consent was obtained from all patients need localizing from February 2014 to September 2015. The Institutional Review Board of Shanghai Pulmonary Hospital approved this study (IRB No. K16-302). Dislodgement occurred in 18 patients who had undergone preoperative CT-guided hookwire or microcoil placement in our center for localizing SPNs. The indications for metallic mark placement were: (I) the maximal transverse diameter of SPN <30 mm and (II) SPN <40 mm from visceral pleura. The practice of procedures was in accordance with prior reports (9). Upon the completion of marker placement, 0.7 mm CT scan was performed to avoid complications, like massive pneumothorax and metallic marker displacement. Patients in whom dislodgement of localization device occurred during the surgery accepted remedial localization.
Surgical procedures
The patients were initially intubated with double-lumen tubes after general anesthesia. Conventionally, only one or two incisions are adopted for resections. In cases of uniportal video-assisted thoracoscopic surgery (VATS), the incision is commonly 4 cm at the 4th/5th intercostal space along the anterior axillary line. A second incision is thus needed for camera in cases of two-port VATS surgery at the 8th intercostal space of mid-axillary line.
Thoracoscopic wedge resections were first considered when atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and benign nodule were reported in frozen section. However, if frozen section indicated invasive adenocarcinoma (IA), lobectomy would be performed. For peripheral pulmonary nodules or highly-suspected pre-invasive lesions, wedge resections were preferred and the specimens were immediately sent to frozen section lab for evaluation. Segmentectomies or lobectomies, plus lymph node dissections, were performed if the pathology suggested micro-invasive or invasive nature of the lesions (15). Wedge resections were comparatively easy, with staples firing around hook wires. For microcoils, however, palpation of the marker was performed before wedge resections (16).
The remedial localization technique
The remedial localization procedure was initiated when intraoperative dislodgement was seen under the thoracoscope. The core mechanism of this technique was to localize the projection point of nodule on the body surface and visceral pleura using CT series. First, the corresponding body surface projection of the nodule was assessed by reading CT images. Its location was confirmed by the level of CT image and the intersection of intercostals space and certain anatomical lines (such as anterior axillary line, midaxillary line, or midclavicular line, etc.).
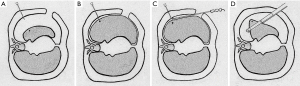
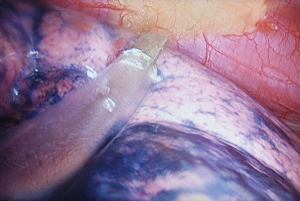
After establishing the body surface projection point, a needle was inserted perpendicularly through the chest wall and the insertion stopped at about 0.2–0.5 cm into the chest cavity under direct thoracoscopic monitoring (Figure 1A). The tip of the needle marked the corresponding visceral pleural projection of the SPN when the lung was inflated again (Figure 1B). Subsequently, a burn mark was made with electrosurgical unit on the visceral pleura, which indicated the position of the nodule on the surface of the lung (Figure 1C). The patient was then transited to one-lung ventilation again, and thoracoscopic wedge resection, which included at least 1 cm away from the burn mark(s), was attempted (Figure 1D). Figure 2 showed remedial localization intraoperatively.
Statistical analysis
The general demographics of patients, including gender, age, and imaging performance of nodules were recorded. Clinical information and pathology results were also recorded. Continuous variables are presented as mean and deviations, and categorical variables are presented as number and percentages.
Results
General information
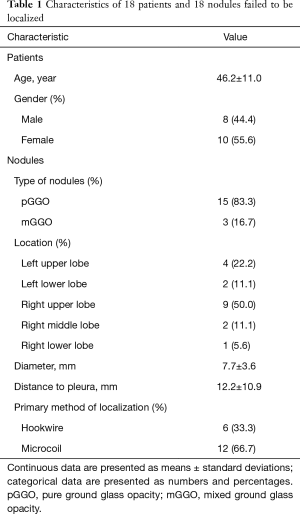
Overall, 18 patients were enrolled in this study. Eight (44.4%) were males and 10 (55.6%) were females, with the mean age of 46.2±11.0 years (Table 1). Among these patients, unhooking occurred in 18 cases during operation. For these unhooked 18 SPNs, 15 (83.3%) were pure ground glass opacity (pGGO), and 3 (16.7%) were mixed ground glass opacity (mGGO). Four (22.2%) SPNs were located in the left upper lobe, 2 (11.1%) in the left lower lobe, 9 (50.0%) in the right upper lobe, 2 (11.1%) in the right middle lobe, and 1 (5.6%) in the right lower lobe. The mean diameter of SPNs was 7.7±3.6 mm. The mean distance from SPN to pleura was 12.2±10.9 mm (Table 1).
Full table
Intraoperative dislodgement
In the present case series, 18 metallic devices dislodged after localizations. In addition to the finding of dislodgement, it was also difficult to locate the primary puncture site on the visceral pleura. Remedial localization and thoracoscopic wedge resections were therefore required. Among these lesions, 6 (33.3%) were initially localized with hookwire and 12 (66.7%) with microcoil.
Results of remedial localization
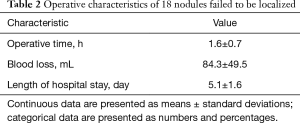
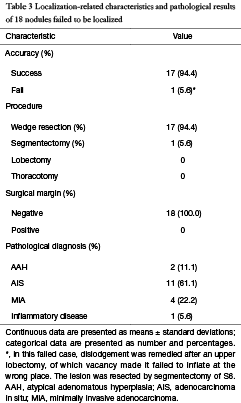
Using the present localization method, 17 SPNs were removed successfully with 94.4% accuracy rate. One (5.6%) SPN was not removed and was converted to segmentectomy of right S6. The median distance between the cautery mark and lesion was 8.2±3.9 mm. The frozen pathology suggested 2 (11.1%) AAH, 11 (61.1%) AIS, 4 (22.2%) MIA, and 1 (5.6%) benign lesion; therefore, no further conversion to segmentectomies or lobectomies was required. The mean operative time was 1.6±0.7 h. The mean intraoperative blood loss was 84.3±49.5 mL. The mean length of hospital stay was 5.1±1.6 days (Table 2). No complications occurred. No postoperative 30-day mortality occurred. Paraffin pathology showed 2 (11.1%) AAH, 11 (61.1%) AIS, 4 (22.2%) MIA, and 1 (5.6%) inflammatory disease (Table 3).
Full table
Full table
Discussion
Along with increasing incidence of operable SPNs, it becomes difficult to localize nodules via palpation. A retrospective trial demonstrated that if the diameter of nodules was ≤10 or >5 mm deeper from visceral pleura during VATS, the probability of palpation failure can be as high as 63% (17). Therefore, the technique of localization has evolved from finger palpation to mechanical marking. Currently, several ways of preoperative localization exist. Hookwire localization has high success rate close to 100%, but also has a high rate of complications, such as pneumothorax, hemorrhage, and dislodgement, among others (5,7,9). Microcoil, another metallic localization method, also has a success rate as high as 98.4%, but high complication rates, such as pneumothorax, hemorrhage, and dislodgement, among others, are also common (7,8,12). Similarly, methylene blue staining has a high rate of success. However, the operation needs to be completed within a very limited time, otherwise the dye diffuses and decreases the accuracy of localization (18). Lipiodol marking has a high success rate of about 99%; however, it may lead to complication of pneumothorax (29%), pulmonary hemorrhage (7%), and spillage (2%) (19). Lipiodol injection can also distort frozen section histological specimens, with a possibility for systemic or pulmonary embolization (20). Intraoperative ultrasonography can localize lung nodules even if ground glass opacity (GGO) is present; however, its limitation is that residual air, operators’ experience, and chronic obstructive pulmonary disease can make localization difficult (21,22).
Despite all the progress of localizing methods, big challenges remain under certain circumstances. For example, one of the most popular localization method, CT-guided percutaneous hookwire or microcoil placement, may be associated with dislodgement (9,12). The underlying reasons of dislodgement are (I) wire extraction due to muscle contraction during patient transportation or (II) loose hooking of the pulmonary tissue. Similar situations may occur with the use of microcoils. When the coil is pushed incompletely into the lung tissue, dislodgement may happen, and the coil may drop into chest cavity (3,23). In clinical practice, thoracic surgeons commonly attempt a tentative wedge resection. If it fails to find the nodule in the specimen, conversions to extended resection may be performed. However, segmentectomy or even lobectomy may not be necessary according to the nature of these tiny nodules.
Thus, it is critically important to re-localize the lesion when the primary method fails. Suzuki (17) suggested that when the distance to the visceral pleura exceeds 10 mm and the diameter of SPN is less than 10 mm, it is almost impossible to locate the nodule by palpation. Furthermore, Ciriaco et al. (24) listed indications for preoperative metallic localization, including (I) SPNs in the 1/3 outer side of the lung; (II) nodules <10 mm and/or >15 mm from the visceral pleural surface; (III) nodule diameter >10 mm and/or between 15–25 mm from the visceral pleural surface; and (IV) no pleural indentation sign. If re-localization is not done during VATS thoracotomy, the problem that was present before localization will become problem again. Therefore, it is important for thoracic surgeons to find a method that would serve as a backup for technical failure of mechanical localization. Otherwise, exceeded lung tissue will be resected or incision will be sutured; accordingly, CT-guided localization will be performed again, leading to ethical problems.
The present study described a practical remedial method for intraoperative localizations of small SPNs in cases of hookwire or microcoil failure. Sometimes, the primary puncture can be localized by the evidence of bleeding or injury (3,11). If not visible, a remedial localization is warranted. Kha et al., for instance, re-localized nodules in their study (3,12). Another way of remedy is extensive resection. Thaete et al. performed lobectomies after dislodgement of invisible localization (25). Li et al. performed a conversion to thoracotomy when invisible dislodgement happened (11). Thaete et al. were far-sighted, and conducting methylene blue localization simultaneously with hookwire localization (25).
Our remedial localization is effective. Out of 18 cases that needed remedy, 17 were treated successfully using scheduled resection. Only one failed. There were four lesions in this patient. One in the right lower lobe needs localizing while the other three were in the right upper lobe with no need of localization. Dislodgement was found, and there was no sign of bleeding. Right upper lobectomy was performed first, and wedge resection was performed after remedial localization. However, no lesion was found in the specimen. Then right lower lobe S6 segmentectomy was performed and the lesion was found in the specimen. Localization in this case failed because during the remedial localization, the right lung was inflated at the wrong place owing to the vacancy of the resected right upper lobe. Thus, for cases with multiple nodules, remedial resection should be tried first before resecting a part of the lung. High success rate of 94.4% demonstrates that our localization is favorable and practical.
Our technique has certain limitations. Although the accuracy is acceptable, present method serves only as the remedial technique. CT-guided hookwire or microcoils placement were associated with good observable accuracy. The presence of a metal mark also helps exceeded resections with an obvious target. As a remedy, present technique narrowed down the search area to about one square centimeter, which was still a bigger area compared to the one with metal marks.
In conclusion, our study is effective and reliable. Remedial localization helped prevent massive resection of lung tissue. When primary mechanical localization fails, present technique offers a good backup plan.
Acknowledgements
Funding: Application of indigenous endoscopic stapler in lung surgery (14DZ1941308).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board of Shanghai Pulmonary Hospital approved this study (IRB No. K16-302). Informed consent was obtained from all patients need localizing from February 2014 to September 2015.
References
- Tamura M, Oda M, Fujimori H, et al. New indication for preoperative marking of small peripheral pulmonary nodules in thoracoscopic surgery. Interact Cardiovasc Thorac Surg 2010;11:590-3. [Crossref] [PubMed]
- Hsu HH, Shen CH, Tsai WC, et al. Localization of nonpalpable pulmonary nodules using CT-guided needle puncture. World J Surg Oncol 2015;13:248. [Crossref] [PubMed]
- Su TH, Fan YF, Jin L, et al. CT-guided localization of small pulmonary nodules using adjacent microcoil implantation prior to video-assisted thoracoscopic surgical resection. Eur Radiol 2015;25:2627-33. [Crossref] [PubMed]
- Xu X, Yao Y, Shen Y, et al. Clinical analysis of percutaneous computed Tomography-Guided hook wire localization of 168 small pulmonary nodules. Ann Thorac Surg 2015;100:1861-7. [Crossref] [PubMed]
- Yoshida Y, Inoh S, Murakawa T, et al. Preoperative localization of small peripheral pulmonary nodules by percutaneous marking under computed tomography guidance. Interact Cardiovasc Thorac Surg 2011;13:25-8. [Crossref] [PubMed]
- Finley RJ, Mayo JR, Grant K, et al. Preoperative computed tomography-guided microcoil localization of small peripheral pulmonary nodules: a prospective randomized controlled trial. J Thorac Cardiovasc Surg 2015;149:26-31. [Crossref] [PubMed]
- Sui X, Zhao H, Yang F, et al. Computed tomography guided microcoil localization for pulmonary small nodules and ground-glass opacity prior to thoracoscopic resection. J Thorac Dis 2015;7:1580-7. [PubMed]
- Sa YJ, Kim JJ, Du Kim Y, et al. A new protocol for concomitant needle aspiration biopsy and localization of solitary pulmonary nodules. J Cardiothorac Surg 2015;10:104. [Crossref] [PubMed]
- Hanauer M, Perentes JY, Krueger T, et al. Pre-operative localization of solitary pulmonary nodules with computed tomography-guided hook wire: report of 181 patients. J Cardiothorac Surg 2016;11:5. [Crossref] [PubMed]
- Ichinose J, Kohno T, Fujimori S, et al. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg 2013;96:1203-8. [Crossref] [PubMed]
- Li W, Wang Y, He X, et al. Combination of CT-guided hookwire localization and video-assisted thoracoscopic surgery for pulmonary nodular lesions: Analysis of 103 patients. Oncol Lett 2012;4:824-8. [PubMed]
- Kha LC, Hanneman K, Donahoe L, et al. Safety and efficacy of modified preoperative lung nodule microcoil localization without pleural marking: a pilot study. J Thorac Imaging 2016;31:15-22. [Crossref] [PubMed]
- Lang-Lazdunski L. Surgery for nonsmall cell lung cancer. Eur Respir Rev 2013;22:382-404. [Crossref] [PubMed]
- Xue Y, Wang YY, Zhang K, et al. A study of complete Video-Assisted thoracoscopic surgery lobectomy in treatment of elderly patients with Non-Small cell lung cancer: curative effect and impact on clinical prognosis. Cell Biochem Biophys 2015;73:399-404. [Crossref] [PubMed]
- Xie D, Wang H, Fei K, et al. Single-port video-assisted thoracic surgery in 1063 cases: a single-institution experience† Eur J Cardiothorac Surg 2016;49:i31-6. [Crossref] [PubMed]
- Dai C, Ren Y, Xie H, et al. Clinical and radiological features of synchronous pure ground-glass nodules observed along with operable non-small cell lung cancer. J Surg Oncol 2016;113:738-44. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Shentu Y, Zhang L, Gu H, et al. A new technique combining virtual simulation and methylene blue staining for the localization of small peripheral pulmonary lesions. BMC Cancer 2014;14:79. [Crossref] [PubMed]
- Kim YD, Jeong YJ. Localization of pulmonary nodules with lipiodol prior to thoracoscopic surgery. Acta Radiol 2011;52:64-9. [Crossref] [PubMed]
- Miura H, Yamagami T, Tanaka O, et al. CT findings after lipiodol marking performed before video-assisted thoracoscopic surgery for small pulmonary nodules. Acta Radiol 2016;57:303-10. [Crossref] [PubMed]
- Kondo R, Yoshida K, Hamanaka K, et al. Intraoperative ultrasonographic localization of pulmonary ground-glass opacities. J Thorac Cardiovasc Surg 2009;138:837-42. [Crossref] [PubMed]
- Matsumoto S, Hirata T, Ogawa E, et al. Ultrasonographic evaluation of small nodules in the peripheral lung during video-assisted thoracic surgery (VATS) Eur J Cardiothorac Surg 2004;26:469-73. [Crossref] [PubMed]
- Pittet O, Christodoulou M, Pezzetta E, et al. Video-assisted Thoracoscopic Resection of a Small Pulmonary Nodule after Computed Tomography–guided Localization with a Hook-wire System. World J Surg 2007;31:575-8. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Thaete FL, Peterson MS, Plunkett MB, et al. Computed tomography-guided wire localization of pulmonary lesions before thoracoscopic resection: Results in 101 cases. J Thorac Imaging 1999;14:90-8. [Crossref] [PubMed]


