Clinical and pathological aspects of microscopic thymoma with myasthenia gravis and review of published reports
Introduction
Thymic epithelial tumors are rare neoplasms. The incidence of thymoma, which comprise the majority of thymic epithelial tumors, is 0.15 per 100,000 person-years (1,2). An interesting biological feature of thymoma is their frequent association with various autoimmune diseases such as y myasthenia gravis. However, the biological mechanisms for the association between thymomas and immunological disorders have not been fully elucidated.
Microscopic thymomas were first described by Rosai et al. in 1976 (3); only 13 cases have been reported to date (4-9). Microscopic thymomas are defined as epithelial proliferations smaller than 1 mm in diameter and characteristically occur in patients with myasthenia gravis without macroscopic thymic epithelial tumors. We here evaluate five such tumors that were found incidentally after thymectomy that was preformed because of deterioration in symptoms of myasthenia gravis. It is important to perform thorough microscopic examination of all thymic tissue resected from patients with myasthenia gravis to maximize detection of microscopic thymomas. The purpose of this article was to elucidate the clinical and pathological aspects of microscopic thymoma and review relevant published reports.
Methods
This retrospective study included five consecutive patients who had been managed at the Division of Chest Surgery of Fukushima Medical University from April 2007 to March 2016. These five patients had undergone extended thymectomies for myasthenia gravis and been found by histopathological examination of the excised specimens to have microscopic thymomas that had not been diagnosed preoperatively. During the same period, 32 extended transsternal thymectomies had been performed on patients with myasthenia gravis at our institution, including these five cases. The 32 cases were divided into the following three groups: microscopic thymoma group (Group M), thymoma group (Group T) and non-thymic tumor group (Group N). Group N does not include any other neoplasm except hyperplasia. The five cases in Group M comprised three men and two women aged 31 to 64 years (mean age 53.8±14.2 years). These patients’ Eastern Cooperative Oncology Group (ECOG) scores were 0 to 1. The Ethics Committee of Fukushima Medical University approved this study. The protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The excised specimens of all 32 patients who had undergone extended thymothymectomies/thymectomies for myasthenia gravis were formalin fixed, embedded in paraffin, sectioned at 5 µm, stained with routine hematoxylin and eosin, and subjected to thorough complete histological examination. Clinicopathological factors were compared between groups using Student’s t-test. All tests were two-sided and a P value <0.05 was considered significant. Statistical analysis was performed with SPSS version 21.0 software (SPSS, Chicago, IL, USA).
Results
Five of the 32 patients who had undergone extended thymectomies for myasthenia gravis were found to have microscopic thymomas, the incidence of which was thus 15.2%. The clinical features of all 32 cases are summarized in Table 1. Preoperative serum anti-acetylcholine receptor antibody (anti-AchR Ab) titers of all five patients in Group M were high (74.4±53.3 nmol/L) and all decreased significantly after surgery (11.7±13.5 nmol/L, P=0.037). The mean pre- and post-operative anti-AchR Ab titers were 74.4±53.3 and 11.7±13.5 nmol/L in Group M, 26.5±30.5 and 9.6±15.2 nmol/L in Group T, and 368±709 and 89.6±164.5nmol/L in Group N, respectively. Remarkably, the mean anti-AchR Ab titer of Group M was significantly higher than that of Group T (P=0.034), as was the mean anti-AchR Ab titer of Group N (P=0.005). Post-operative pathological examination of resected specimens revealed that all five patients with microscopic thymomas had multifocal lesions; additionally, one of them had lymphoid hyperplasia. All these five cases had type A thymomas according to the World Health Organization (WHO) classification. Figure 1 is a representative photomicrograph showing the typical histological features of microscopic thymoma.
We have experienced just one recurrent case after surgery in group M. The case had a recurrence after 5 years.
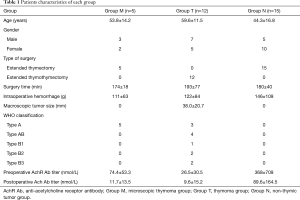
Full table

Discussion
Myasthenia gravis is often associated with pathological changes in the thymus, either lymphoid hyperplasia (15–85%) or thymoma (10–15%) (3). Myasthenia gravis is associated with abnormal activation of thymic B lymphocytes resulting in lymphoid hyperplasia or of the epithelial cells resulting in thymoma. The randomized trial of thymectomy in myasthenia gravis (MGTX study) had clarified that extended thymectomy improved clinical outcomes in patients with nonthymomatous myasthenia gravis (4).
Microscopic thymomas characteristically occur in patients with myasthenia gravis (5). The morphology of microscopic thymoma differs from that of conventional thymoma. According to the current WHO classification, microscopic thymomas are defined as epithelial proliferations of less than 1 mm in diameter. They are usually multifocal, located in the cortex or medulla, and preferentially occur in patients with myasthenia gravis without macroscopically evident tumor (5,8,10). “Nodular hyperplasia” would be a more appropriate term for these lesions because they present as small thymic epithelial islands that are found incidentally and lack the morphological features of conventional thymomas such as lobulation, perivascular spaces, immature T cells, and medullary differentiation (8,11-13). That microscopic thymomas occur in the cortical or medullary regions and are frequently multifocal suggests that multiple lesions originate from distinct epithelial clones present in different areas of the thymus (5). Microscopic thymomas are considered to be incipient thymomas or precursor of thymoma; however, these possibilities remain unproven. A review of published reports in English revealed only six studies (5-9,14); we have summarized the clinical characteristics of the resultant total of 18 cases, including our cases, in Table 2. In four of our cases and in nine of the 13 reported cases, the microscopic thymomas were not associated with lymphoid hyperplasia of the thymus, suggesting there is a relationship between microscopic thymoma and myasthenia gravis (5). In our institution, 5 of the 32 patients who had undergone extended thymothymectomies/thymectomies for myasthenia gravis during the study period were found to have microscopic thymomas, making the incidence 15.2%. This is comparable to data published by Vaideeswar et al. and Pescarmona et al., who reported incidences of 3.8% and 15%, respectively (5,6). The incidence may be higher if extended thymectomies are performed on patients with myasthenia gravis and the entire resected specimens are evaluated pathologically.
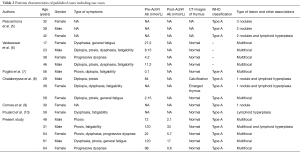
Full table
Similar to the interesting phenomenon of microscopic thymoma, some authors have reported mediastinal ectopic thymic tissue in patients with and without thymic disease or myasthenia gravis. According to these reports, the frequency of mediastinal ectopic thymic tissue is high. Sanei et al. (15) reported finding ectopic thymic tissue in 70.85% of patients without myasthenia gravis or thymic disease and that this is distributed through the mediastinum as follows: left pericardiophrenic 50%, right pericardiophrenic 31.9%, aortopulmonary window 19.4%, and aortocaval groove 12.5%. Zielinski et al. (16) reported detecting ectopic thymic tissue in 56.9% of patients with myasthenia gravis. Ambrogi et al. (17) and Mineo et al. (18) recently reported incidences of ectopic thymic tissue in the mediastinum in patients with myasthenia gravis of 67% and 80%, respectively. Because the incidence of ectopic thymic tissue is so high, the thymic tissue and perithymic fat tissue should be completely removed and carefully evaluated; microscopic thymomas can occur in ectopic thymic tissue, especially in the pericardiophrenic area bilaterally. We usually perform median sternotomy for the treatment of myasthenia gravis patients in order to achieve complete resection of thymic tissue for better outcomes.
The microscopic thymomas were multifocal in all 5 cases (100%) in Group M and in 11/18 previously reported cases (61.1%), indicating that these lesions can occur simultaneously in all regions of the thymus. All our five cases and all those previously reported were type A thymomas according to the WHO classification. Furthermore, three of the patients in Group T had type A thymomas, even though this histological type is not thought to be associated with myasthenia gravis (19). Thymic carcinoma is also not considered to be associated with myasthenia gravis; in our study, none of the patients with myasthenia gravis had thymic carcinoma. The recurrence in Group M, was amazing because microscopic thymoma was included into type A thymoma according to WHO classification. The pathology of recurrent lesion showed type B2 thymoma
In this study, serum anti-AchR Ab titers were significantly higher preoperatively than postoperatively in all groups, indicating that extended thymectomy is a valid means of managing myasthenia gravis whether or not macroscopic thymomas are detected on CT scan images before surgery (20). Furthermore, median anti-AchR Ab titers were significantly higher in Group M and Group N than in Group T, indicating that production of anti-AchR Ab may not depend on tumor size.
We have here reported some aspects of microscopic thymomas, such as their incidence, significant changes in anti-AchR Ab titers before and after surgery, and their pathological characteristics. However, even including previously published data, there were too few cases to draw definite conclusions. Large multicenter studies are required to elucidate aspects of this mysterious disease. Thoracic surgeons and pathologists must be educated to search thoroughly for microscopic thymomas lesions throughout resected specimens. Furthermore, microscopic thymomas may be misdiagnosed as microthymomas (21) which differ from microscopic thymomas. Therefore, we consider a distinctive name such as “nodular hyperplasia of the thymic epithelium” would be preferable to “microscopic thymoma”.
In conclusion, microscopic thymomas are frequently multifocal, sometimes associated with lymphoid hyperplasia, and tend to be type A thymomas according to the WHO classification. In all of our cases, Anti-AchR Ab titers decreased significantly after surgery, including in Group M. We recommend wide excision of the thymus gland and all surrounding adipose tissue in patients with myasthenia gravis and complete histological examination of all macroscopic portions of the excised tissue to enable more detailed assessment of the incidence, causes, pathogenesis, and functional significance of microscopic thymomas.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Ethics Committee of Fukushima Medical University approved this study. The protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
References
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003;105:546-51. [Crossref] [PubMed]
- Rosai J, Levine GD. Tumors of the thymus. In: Atlas of tumor pathology. 2nd series, fascicle 13. Washington, DC: Armed Forces Institute of Pathology, 1976.
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Pescarmona E, Rosati S, Pisacane A, et al. Microscopic thymoma: histological evidence of multifocal cortical and medullary origin. Histopathology 1992;20:263-6. [Crossref] [PubMed]
- Vaideeswar P. Microscopic thymoma: a report of four cases with review of literature. Indian J Pathol Microbiol 2011;54:539-41. [Crossref] [PubMed]
- Puglisi F, Finato N, Mariuzzi L, et al. Microscopic thymoma and myasthenia gravis. J Clin Pathol 1995;48:682-3. [Crossref] [PubMed]
- Chalabreysse L, Orsini A, Vial C, et al. Microscopic thymoma. Interact Cardiovasc Thorac Surg 2007;6:133-5. [Crossref] [PubMed]
- Cornea R, Lazăr E, Dema A, et al. A nodular hyperplasia of the thymic epithelium (so-called microscopic thymoma). Rom J Morphol Embryol 2009;50:729-31. [PubMed]
- Travis WD, Brambilla E, Müller-Hermelink HK, et al. Pathology and genetics of tumors of the lung, pleura, thymus and heart. Lyon: IARC Press, 2004;145-97.
- Rosai J. Rosai and Ackerman’s Surgical Pathilogy. 8th ed. Edinburgh: Mosby, 2004: 459-514.
- Cheuk W, Tsang WY, Chan JK. Microthymoma: definition of the entity and distinction from nodular hyperplasia of the thymic epithelium (so-called microscopic thymoma). Am J Surg Pathol 2005;29:415-9. [Crossref] [PubMed]
- Mori T, Nomori H, Ikeda K, et al. Microscopic-sized "microthymoma" in patients with myasthenia gravis. Chest 2007;131:847-9. [Crossref] [PubMed]
- Poulard G, Mosnier JF, Dumollard JM, et al. Microscopic thymoma and myasthenia gravis. Ann Pathol 1994;14:203-4. [PubMed]
- Sanei B, Tabatabie SA, Bigdelian H, et al. Distribution of mediastinal ectopic thymic tissue in patients without thymic disease. Adv Biomed Res 2015;4:18. [PubMed]
- Zieliński M, Kuzdzal J, Szlubowski A, et al. Comparison of late results of basic transsternal and extended transsternal thymectomies in the treatment of myasthenia gravis. Ann Thorac Surg 2004;78:253-8. [Crossref] [PubMed]
- Ambrogi V, Mineo TC. Active ectopic thymus predicts poor outcome after thymectomy in class III myasthenia gravis. J Thorac Cardiovasc Surg 2012;143:601-6. [Crossref] [PubMed]
- Mineo TC, Ambrogi V. Outcomes after thymectomy in class I myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:1319-24. [Crossref] [PubMed]
- Okumura M, Shiono H, Minami M, et al. Clinical and pathological aspects of thymic epithelial tumors. Gen Thorac Cardiovasc Surg 2008;56:10-6. [Crossref] [PubMed]
- Okumura M, Ohta M, Takeuchi Y, et al. The immunologic role of thymectomy in the treatment of myasthenia gravis: implication of thymus-associated B-lymphocyte subset in reduction of the anti-acetylcholine receptor antibody titer. J Thorac Cardiovasc Surg 2003;126:1922-8. [Crossref] [PubMed]
- Cheuk W, Tsang WY, Chan JK. Microthymoma: definition of the entity and distinction from nodular hyperplasia of the thymic epithelium (so-called microscopic thymoma). Am J Surg Pathol 2005;29:415-9. [Crossref] [PubMed]


