The use of thrombolytics in the management of complex pleural fluid collections
Introduction
Pneumonia accounts for 1.3 million hospitalizations annually, and of those admissions, 36 to 66 percent (%) have pleural effusions (1). Patients are typically treated with antibiotics, drainage of the pleural fluid, and either intrapleural administration of fibrinolytic agents or operative drainage (2). Fibrinolytic agents are intended to break up loculations in the infected pleural space and allow re-expansion of the lung (1,2). Mortality increases from 7% to 33% as effusions evolve, leading to progressive sepsis and shock (1). We report the use of alteplase in the pleural space to decrease hospital stay, surgical interventions, and complications. The objective of the study is to determine the efficacy of thrombolytics for the management of complex pleural fluid collections.
Methods
An institutional review board (IRB) approved retrospective chart review was performed of all consecutive patients treated at a single institution from July 01, 2007 to November 01, 2012. The IRB ID number was 5130004. The study was retrospective review and a waiver of informed consent was approved by the IRB.
We examined all patients treated with alteplase for loculated pleural fluid collections. Baseline patient demographics, co-morbidities, smoking history, antibiotic use, alteplase dose, number of days utilizing alteplase therapy, and chest tube output after instillation were recorded. All pleural fluid collections were class 5 or greater as described by Light (3); although, not all effusions were related to infection. Treatment endpoints were: (I) days to resolution of pleural fluid collection; (II) time to surgery; and (III) mortality.
The protocol is: 6 mg of alteplase in 50 mL of normal saline instilled via a pleural chest tube. The chest tube is clamped for 4 hours (dwell time); then, unclamped and allowed to drain. One dose was given per 24 hour period, for a total of three doses. Patients were evaluated with plain chest radiographs (CXR) and chest computed tomography (CT) prior to alteplase therapy and a CXR was performed daily prior to repeat additional instillations. Patients who showed complete clearance on the CXR after any dose were considered to be adequately treated and additional doses of alteplase were not given. Patients with a persistent opacification on CXR after the third dose had been drained, underwent a CT scan of the chest. Success of treatment was determined by resolution or near resolution of the fluid collection(s) on CT chest imaging. Patients with persistent loculated effusions were considered to have failed medical treatment and underwent decortication. Persistent loculated effusions were determined based on the surgeons’ analysis and read of the CT chest imaging. If the effusion was equal to the size of the image prior to administration of the effusion, it was considered unresolved and surgery was indicated. If the effusion was significantly large, though, decreased in size from the initial effusion, it was considered unresolved and surgery was indicated. Surgery was not indicated if the effusion was significantly resolved, based on the surgeon read of the imaging. The chest tube used ranged from 8.5, 28 and 32 F chest tubes depending on surgeon preference and size of the pleural effusion.
Statistical analysis was performed utilizing SAS software to determine factors associated with failure of alteplase therapy. Patient factors such as co-morbidities, history of recent trauma, prior lung disease, history of lung cancer, and smoking history were examined utilizing t-test and statistical significance was assumed if P<0.05.
Results
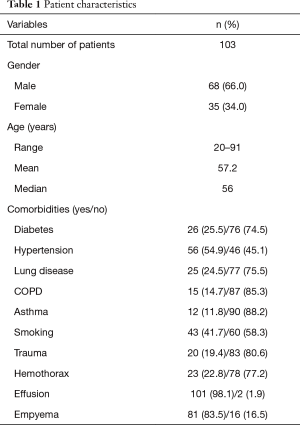
One hundred and three [103] consecutive patients were identified who received alteplase therapy for management of loculated pleural fluid collections. The age of the patients ranged from 20–91 y (mean 57.2 y); 68 (66%) were male. The most common co-morbidity was hypertension (55%, n=60), and 35% (n=32) of patients had a history of smoking. Additionally, 8 (7.7%) of patients had a previous history of lung cancer, and 7 (6.8%) had a history of lung surgery. Of patients with loculated pleural fluid collections, 20 (18.2%) had a history of recent trauma (Tables 1,2).
Full table
Full table
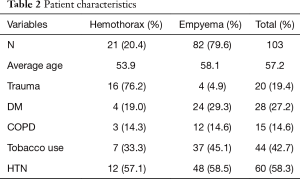
Eight-two patients (79.6%) had a loculated pleural fluid collection secondary to an empyema, and 21 (20.4%) had a retained hemothorax. Of the 21 patients with a retained hemothorax, 16 were secondary to blunt trauma with rib fractures as the most common injury in 14 of the 16 trauma patients. Penetrating trauma and malignant hemothorax accounted for the remainder of the retained hemothoraces. A third of the patients with a retained hemothorax became secondarily infected. Three of the 21 patients with retained hemothoraces died, caused by empyema, aspiration pneumonia, and massive hemothorax.
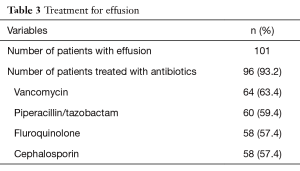
Ninety-six patients (93.2%) were treated with antibiotics; 7 patients were not treated with antibiotics, and all had a diagnosis of a retained hemothorax. The most common antibiotics used were vancomycin (n=64), piperacillin/tazobactam (n=60), fluoroquinolones (n=58), and cephalosporins (n=58); 86 patients were prescribed more than one antibiotic (Table 3).
Full table
The average length of stay (LOS) from day of admission to discharge for was 25.9 days (3–143 days). The average number of days in the hospital for the diagnosis of a hemothorax and empyema was 21.9 and 26.9 days respectively (Table 3). Forty patients (36.4%) had follow-up visits, with a mean time to follow of 12 days (range, 2–28 days). For the 103 patients reviewed, there were 110 hemothoraces with installments of alteplase via the chest tube. The average time from diagnosis to alteplase treatment for a hemothorax was 12.8 days (range, 1–32 days); and 16.2 days (range, 4–48 days) for an empyema. Patients with a history of lung disease had a statistically significant shorter time from diagnosis to TPA therapy; mean 9.4 days compared to 16.3 days for patients without lung disease (P=0.009).
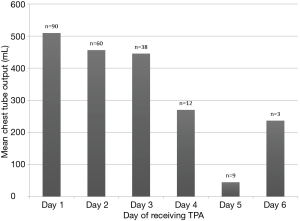
The mean number of days on alteplase therapy was 2.2±1.4 days, (range, 1–11 days). The average chest tube output after day 1 was 509.8, after day 2 was 456.6 and after day 3 was 445.8 (Figure 1). Eighty-seven (79.1%) had their chest tube removed prior to discharge. The average time from alteplase completion to chest tube removal was 5.7±4.5 days (range, 1–21 days). Eleven patients (10.7%) underwent decortication; of which 5 had a retained hemothorax and 6 had a loculated pleural effusion. After completion of alteplase therapy, the average time to discharge was 10.2 days (range, 1–109 days). The average time from completion of alteplase therapy to discharge in the hemothorax and empyema groups was 6.9 and 11.1 days, respectively (Table 4).
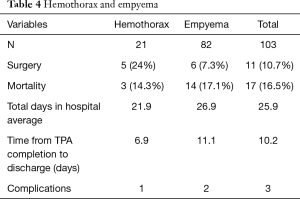
Full table
Seventeen patients (16.5%) died; 3 patients (14.3%) died in the hemothorax group, while 14 patients (17.1%) died in the empyema group. The cause of death was: sepsis (4), respiratory failure (5), aortic injury causing hemorrhage (1), cardiac arrest (1), aspiration (1), and progression of malignancy (1). The patient who died of an aortic injury presented as trauma patient with a massive left hemothorax after a high speed motor vehicle collision. The patient developed a retained hemothorax; TPA was administered and the chest tube drained 2.5 liters of sanguineous fluid. The patient was transferred to the intensive care unit. The patient had a cardiac arrest in the intensive care unit and was emergently taken to the operating room for an exploratory left thoracotomy. Three liters of bright red blood and clots was evacuated from the chest. The autopsy demonstrated an undiagnosed aortic laceration secondary to rib fractures of ribs 9 and 10. The hematoma was stable for 9 days and the patient became unstable after the administration of TPA. The TPA probably removed the clotted blood that had caused a tamponade of the injury. He died in the operating room.
Discussion and conclusions
Fibrinolytics have been used in the intrapleural space as a means to break up the loculations that occur as the physiological response to parapneumonic effusions and allow re-expansion of the lung (1,2,6-9). Inflammation and the presence of mesothelial cells in the pleural space bring about the fibrinopurulent phase, where the effusion becomes infected and there is an imbalance between coagulation and fibrinolysis (5,6). Fibrinolysis is inhibited, causing deposits to occur along the pleura, resulting in loculations and an empyema (6).
Fibrinolytics such as streptokinase and urokinase were the first agents used to activate the fibrinolytic system and activate plasminogen directly (3,5-7,10,11). These actions allow for the dissolution of fibrin complexes present in the parapneumonic effusion or empyema (6). TPA acts to break up the fibrinous debris, allowing drainage of fluid (5). The half-life of TPA in the blood is only 6 minutes and is undetermined in the pleural space, therefore the appropriate dwell, amount, and timing of TPA is still undetermined (6).
The efficacy of fibrinolytics in loculated pleural effusions and empyemas has been questioned in the literature. A double blind randomized study by Maskell and colleagues in the New England Journal of Medicine found that intrapleural administration of streptokinase does not improve mortality, the rate of surgery, or the length of hospital stay (2,6). This study randomized patients to placebo or control groups, with control groups receiving 250,000 IU of streptokinase diluted in 30 mL of normal saline delivered into the pleural space every 12 hours for 6 doses total. There was a slightly higher number of adverse events in the streptokinase group in Maskell’s study, but this was not a statistically significant difference (P=0.08) (2).
A Cochran review concluded that fibrinolytic therapy showed benefit by reducing need for surgical intervention despite the 2005 trial by Maskell (7). Stefanutti et al. reported success with intrapleural instillation of urokinase in pediatric patients using weight adjusted doses (8). Only 4 of 41 patients required further operative treatment for parapneumonic effusions (8). Furthermore, Thommi and colleagues found that alteplase was significantly more effective than placebo in reducing surgical interventions in patients with empyemas (1). In this study, 95 percent of patients received adequate drainage and treatment, compared to 12 percent in the placebo group (1).
Froudarakis and colleagues performed a prospective study in twenty patients with intrapleural administration of recombinant TPA for parapneumonic effusions or persistent pleural effusions (5). They found a 95% success rate with the use of intrapleural TPA, with improved drainage after instillation, decrease in inflammatory mediators such as white blood cells and CRP, and minimal complications (5). Complications were described as mild pain with instillation and mild, local hemorrhage in 3 of 20 patients (5).
A retrospective review of 118 patients found successful treatment of parapneumonic and pleural effusions in 69.5 percent of patients treated with one to eight doses of 25 mg of alteplase diluted in 120 mL of saline and instilled into the intrapleural space. Intrapleural alteplase was successful in 78.1 percent of empyemas, 61.5 percent of patients with hemothoraces, 100 percent of those with malignancy as a cause of the pleural process, 71.4 percent of complex pleural effusions, and 48 percent of parapneumonic effusions. Success was determined by resolution or near resolution of the fluid collection on imaging with avoidance of surgical intervention. The overall effectiveness of alteplase was 86.4 percent, with the highest success rate noted after one or two doses. Patients who required more than two doses were more likely to have residual disease, suggesting operative intervention should be considered after two doses. Unsuccessful treatment occurred in 35 patients, seven of these patients experienced bleeding requiring operative intervention. Bleeding was associated with empyema in 6.3 percent, malignancy in 12.5 percent, complex pleural effusion in 4.8 percent, and parapneumonic effusion in 8 percent (9). This comparatively high rate of bleeding may result from higher dosing used in that study.
Rehman and colleagues (12) randomized patients to receive placebo, TPA, DNase or TPA plus DNase. They found no difference between placebo and TPA with respect to avoiding surgical deloculation. When DNase was added to TPA, only 4% were referred for surgical intervention when followed over a three month period. A 1 hour dwell time with 10 mg of TPA was used twice daily (12).
Surgical management remains an option in the management of empyema and at one point was considered the gold standard (13). Chen and colleagues reviewed thoracoscopic management of 101 pediatric empyemas in their institution (4). In their study the median duration of time from diagnosis of empyema to surgical management was 4 days, however, 35.6 percent first underwent thoracostomy tube placement followed by thoracoscopic management. The median duration of chest tube duration was 6 days with a range between 1 and 28 days. Chen and colleagues noted increased difficulty with surgery when a chest tube was placed preoperatively, noted by statistically significant increased operative time in the thoracostomy tube group (94 versus 72 minutes). Primary treatment with thoracoscopy was performed in 65 percent of patients. In this study 32.7 percent were treated in the intensive care unit preoperatively, which was associated with a prolonged hospital stay. The median duration of hospital stay postoperatively in this study was 13 days (4).
St Peter and colleagues performed one of the first prospective studies comparing thoracoscopic and intrapleural fibrinolytics in children (13). In this study 18 children were randomized to fibrinolysis versus surgical management. The results showed similar outcomes in regards to post treatment LOS, days of oxygen support, time to defervescence, and analgesia. A statistically significant difference was found between the hospital charges between the patient groups. The surgical group averaged $11,700 in hospital charges versus $7,600 for the fibrinolysis group. Only three patients (16.6 percent) failed fibrinolytic therapy in this study, requiring surgical thoracoscopy for definitive management. In the surgical group, two of the patients became clinically worse, requiring ventilator support, progression of sepsis, and requiring temporary hemodialysis, while no progression of disease occurred in the fibrinolytic group (13).
Sonnappa et al. had similar results in their prospective randomized trial for children with empyema, comparing thoracoscopic decortication compared to intrapleural fibrinolysis with urokinase (10). There was no difference in time to discharge after intervention between the fibrinolytic and surgical group. Their surgical group were able to remove the chest drain 1 day earlier than the fibrinolytic group (P=0.055). Only 2 of the patients in the fibrinolytic therapy group required thoracoscopic surgery for definitive management. The surgical group had a 25 percent increase in cost compared to the fibrinolytic therapy group, similar to the findings of St Peter (10,11).
Surgical therapy is not without risks. In elderly patients with multiple co-morbidities, mortality can be up to 5 percent in thoracoscopic surgery and 10 percent with decortication. Recurrence of empyema can occur in up to 2.6 percent of patients treated surgically. Complications can occur, including bleeding, infection, intolerance to single lung ventilation, re-expansion pulmonary edema, prolonged ventilation, intercostal neuralgia, air leaks, and bronchopleural fistulas. Postoperative pain can cause additional problems, especially in the elderly patients (1).
In our study, the average hospital stay was 25.9 days, with the diagnosis of loculated pleural fluid collection or empyema was diagnosed later in the hospital course. The delay in treatment, was secondary to all of these patients were referred and the thoracic surgery service was the consulting service. As the treatment protocol was refined, and conversations were had with the referring services, the time to referral for treatment decreased. Only 10.7 percent of these patients failed thrombolytic treatment, requiring surgical decortication. This is inferior to the results of Rehman et al. when DNase was added but far superior to their results for TPA alone. They found that only 4% of patients were referred for surgery. This was when patients were followed over three months. We referred no patients for surgery after hospital discharge. They found no difference from placebo when TPA was used alone. This may have been related to their short dwell time, implying that dwell time may be more important than higher doses, 10 mg, in this case, more frequent administration and perhaps even the addition of DNase. Their results may also have been adversely affected by their reliance on changes in plain radiography to assess effectiveness. In our experience, plain radiography is insufficiently sensitive and can lead to confusion between residual effusion and residual parenchymal consolidation. In our study, TPA led to near complete resolution of the effusion when CT was used to evaluate effectiveness. We also utilized a much more truncated time schedule, referring patients for surgery after failure at three days, rather than over a period of three months.
Those patients with a known history of lung disease had a statistically significant earlier time to diagnosis and initiation of fibrinolytic therapy. Those patients may have been monitored more closely because of their known comorbidity. Overall, the data shows that a significant number of patients with class 5–7 pleural effusions and empyemas can a benefit from intrapleural fibrinolytic therapy and that all such patients warrant a trial of intrapleural thrombolytics prior to committing to surgical intervention. This approach may reduce the cost and morbidity.
The variability in outcomes of intrapleural fibrinolytics in the literature can be attributed to inconsistent study characteristics (6). For example, the criteria for the use of fibrinolytics, dose, timing of the doses, variation of chest tubes, and indications for surgery were not standardized in many of the studies. In addition, patients who received intrapleural fibrinolytics in many of the prior studies may have been poor surgical candidates who would not have survived a surgical treatment, such as thoracoscopic or open thoracotomy approach.
We present the largest, consecutive series to date of complex pleural effusions treated with alteplase. We utilized a low dose with once daily administration that did not result in any bleeding events. This was associated with a very low proportion of patients requiring salvage decortication. Fibrinolytic therapy has been associated with lower hospital costs, decreased pain, and minimal complications. Some patients may not respond to treatment with fibrinolytic therapy, and at that point surgical therapy may be appropriate for further treatment. Further studies are needed to determine the effect of dose, dwell time and frequency of administration on outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by an institutional review board (No. 5130004) and a waiver of informed consent was approved by the IRB.
References
- Thommi G, Shehan JC, Robison KL, et al. A double blind randomized cross over trial comparing rate of decortication and efficacy of intrapleural instillation of alteplase vs placebo in patients with empyemas and complicated parapneumonic effusions. Respir Med 2012;106:716-23. [Crossref] [PubMed]
- Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005;352:865-74. [Crossref] [PubMed]
- Light RW. A new classification of parapneumonic effusions and empyema. Chest 1995;108:299-301. [Crossref] [PubMed]
- Chen JS, Huang KC, Chen YC, et al. Pediatric empyema: outcome analysis of thoracoscopic management. J Thorac Cardiovasc Surg 2009;137:1195-9. [Crossref] [PubMed]
- Froudarakis ME, Kouliatsis G, Steiropoulos P, et al. Recombinant tissue plasminogen activator in the treatment of pleural infections in adults. Respir Med 2008;102:1694-700. [Crossref] [PubMed]
- Hamblin SE, Furmanek DL. Intrapleural tissue plasminogen activator for the treatment of parapneumonic effusion. Pharmacotherapy 2010;30:855-62. [Crossref] [PubMed]
- Cameron R, Davies HR. Intra-pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema. Cochrane Database Syst Rev 2008.CD002312. [PubMed]
- Stefanutti G, Ghirardo V, Barbato A, et al. Evaluation of a pediatric protocol of intrapleural urokinase for pleural empyema: a prospective study. Surgery 2010;148:589-94. [Crossref] [PubMed]
- Ben-Or S, Feins RH, Veeramachaneni NK, et al. Effectiveness and risks associated with intrapleural alteplase by means of tube thoracostomy. Ann Thorac Surg 2011;91:860-3; discussion 863-4. [Crossref] [PubMed]
- Sonnappa S, Cohen G, Owens CM, et al. Comparison of urokinase and video-assisted thoracoscopic surgery for treatment of childhood empyema. Am J Respir Crit Care Med 2006;174:221-7. [Crossref] [PubMed]
- Gates RL, Hogan M, Weinstein S, et al. Drainage, fibrinolytics, or surgery: a comparison of treatment options in pediatric empyema. J Pediatr Surg 2004;39:1638-42. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- St Peter SD, Tsao K, Spilde TL, et al. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. J Pediatr Surg 2009;44:106-11; discussion 111. [Crossref] [PubMed]