McKeown or Ivor Lewis totally minimally invasive esophagectomy for cancer of the esophagus and gastroesophageal junction: systematic review and meta-analysis
Introduction
The annual incidence of esophageal carcinoma is increasing (1). Esophagectomy remains the cornerstone for curative treatment, most often after neoadjuvant therapy (2). Minimally invasive esophagectomy (MIE), consisting of hybrid minimally invasive esophagectomy (HMIE) or totally minimally invasive esophagectomy (TMIE), has been shown to be superior compared to open esophagectomy regarding perioperative outcome (3,4) without compromising oncologic safety (5,6). This has led to a progressive adoption of MIE and currently, 45% of all patients worldwide with resectable esophageal cancer undergo MIE (7).
Similar to open esophagectomy, MIE can consist of transhiatal esophagectomy (8), McKeown esophagectomy (9) or Ivor Lewis esophagectomy (10), but Ivor Lewis or McKeown procedures are usually performed since they allow adequate thoracic lymph node dissection (11,12). In patients with esophageal tumors above the level of the carina, an Ivor Lewis procedure is unfeasible because it might compromise adequate resection margins. For patients with lower esophageal or gastroesophageal junction tumors, both McKeown and Ivor Lewis procedures are considered to be oncologically feasible. Supposed benefits of cervical anastomosis are that it is technically less challenging than totally minimally invasive intrathoracic anastomosis and that if an anastomotic leak occurs, it can be managed more easily than intrathoracic leakage. However, intrathoracic anastomosis after MIE is believed to be associated with a lower incidence of anastomotic leakage and better functional results.
It is currently unknown whether minimally invasive McKeown or minimally invasive Ivor Lewis esophagectomy should be preferred for these patients and both cervical and intrathoracic anastomoses are performed (7). The aim of this article is therefore to perform a systematic review and meta-analysis of studies comparing minimally invasive McKeown esophagectomy with minimally invasive Ivor Lewis esophagectomy.
Methods
Literature search
The electronic databases of Medline, Embase and the Cochrane Central Register of Controlled Trials databases were searched using the following search terms (and combinations of these terms): minimal invasive, minimally invasive, laparo-thoracoscop*, laparothoracoscop*, thoracolaparoscop* OR, thoraco-laparoscop*, laparoscop*, hybrid, video assisted thoracic surgery (VATS), video-assisted, video assisted, thoracoscop* esophagectom*, oesophagectom*, resection* oesophagus, oesophageal, oesophagal, esophagus, esophageal, esophagal. No language restrictions were applied and all results up to December 2016 were included. Medical ethical approval was not sought because no new patient data was obtained for this study.
Criteria for selecting studies for this review
Comparative cohort studies or randomized controlled trials that included patients undergoing HMIE or TMIE and that compared McKeown versus Ivor Lewis procedures were included. Exclusion criteria were: less than 10 patients in a treatment arm, unclear description of operative technique rendering classification into McKeown or Ivor Lewis procedures impossible and studies that contained more that 10% other procedures in one of the arms (i.e., minimally invasive transhiatal esophagectomy). VATS procedures and hand-assisted laparoscopic surgery (HALS) procedures were regarded as minimally invasive and were also included.
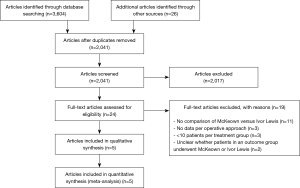
All references of studies were screened on title and abstract by two authors independently (BK and FvW). All studies that were not excluded in the screening stage were assessed in full text for eligibility. If discrepancies occurred, this was discussed in a meeting and if no consensus could be reached, another discussion meeting with a third author (CR) was held until consensus was reached. This process will be described in a flow chart according to the PRISMA statement (13).
Quality assessment
All studies were independently assessed for methodological quality by BK and FvW using the Newcastle-Ottawa rating scale (14). Discrepancies were resolved in discussion. In case of persisting discrepancy, a meeting with a third author (CR) was held and discrepancies were discussed until consensus was reached.
Outcome parameters and data extraction
The primary outcome parameter was anastomotic leakage. Secondary outcome parameters were: all complications, severe complications (CD ≥3), pneumonia, pulmonary complications, chyle leakage, wound infection, recurrent laryngeal nerve (RLN) palsy, benign anastomotic strictures, operating time, blood loss, reoperation rate, reintervention rate, hospital length of stay, ICU length of stay, postoperative mortality (30-, 90-day and in hospital mortality), R0 resection rate, number of lymph nodes found, quality of life and costs. Data was extracted and was entered into review manager (version 5.3). Continuous variables were expressed as median and interquartile ratio or range, the mean and SD were estimated from the available data by methods described elsewhere (15,16).
Analysis
Meta-analysis was performed if data on an outcome parameter was reported in at least two studies in a way that was compatible with meta-analysis. The Mantel-Haenszel method for dichotomous data was used, presented as relative risks (RR) with 95% confidence intervals (CIs). The inverse variance method was used for meta-analysis of continuous data; results are presented as standardized mean difference (SMD) with 95% CIs. The statistical heterogeneity was assessed with I2. In the absence of substantial statistical heterogeneity [I2 ≤50% (15)] a fixed-effect model was used. In case of substantial heterogeneity (I2 >50%), a random-effects model was used. Statistical analyses were performed with Review Manager (version 5.3).
Results
Studies
A summary of the screening and selection process according to PRISMA (13) is shown in Figure 1. No studies comparing McKeown versus Ivor Lewis procedure in patients undergoing HMIE were identified. Five studies with 1,681 patients undergoing TMIE were ultimately included for analysis (17-21). The characteristics of the included studies are summarized in Table 1.
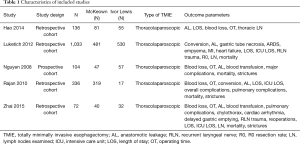
Full table
Quality assessment
The results of the quality assessment of the included studies are shown in Table 2. There were no randomized controlled trials. Studies scored 6 or 7 stars out of 9 according to the Newcastle-Ottawa rating scale, corresponding to a moderate risk of bias. Four studies were retrospective cohort studies and one was a prospective cohort study. One study clearly stated that MIE Ivor Lewis procedures were predominantly performed in a more recent time period (19). This was not described for the other studies.
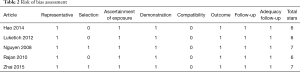
Full table
Heterogeneity
Considerable heterogeneity was found for the outcome parameters pulmonary complications (I2 =73%), intensive care length of stay (I2 =95%), examined lymph nodes (I2 =79%), operating time (I2 =99%) and blood loss (I2 =70%). For these parameters, a random effects model was used. No sensitivity analysis was performed because this was considered unfeasible with only 2 or 3 studies available for the outcome parameters with high heterogeneity.
Meta-analysis
The outcome parameters severe complications (CD ≥3), pneumonia, wound infection, reintervention, quality of life and costs were not reported in any of the included studies. The outcome parameters chyle leakage, reoperation, R0 resection rate were reported, but not enough data was available to perform meta-analysis.
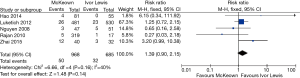
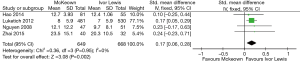
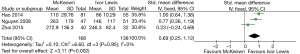
The results of the meta-analysis are shown in Table 3. The incidence of anastomotic leakage was 5.2% after McKeown esophagectomy and 4.7% after Ivor Lewis esophagectomy (RR =1.39, 95% CI =0.90–2.15, P=0.14) (Figure 2). Totally minimally invasive Ivor Lewis esophagectomy was associated with a lower incidence of RLN trauma (RR =6.70, 95% CI =3.09–14.55, P<0.001), a shorter hospital length of stay (SMD =0.17, 95% CI =0.06–0.28, P=0.002) and less blood loss (SMD =0.69, 95% CI =0.25–1.12, P=0.002) compared to totally minimally invasive McKeown esophagectomy (Figures 3-5). There were no significant differences between the groups regarding the other outcome parameters.
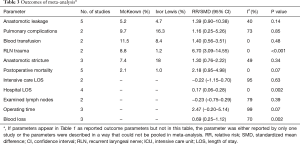
Full table
Conclusions
In this meta-analysis, no difference in anastomotic leakage was found between McKeown and Ivor Lewis esophagectomy in patients undergoing TMIE. The incidence of anastomotic leakage was lower than in recent randomized controlled trials (2,3) and this might be explained by the fact that no standardized definitions of complications were used across studies. For example, the largest study that was included in this meta-analysis only reported anastomotic leakage if a reoperation was required (19). Recently, the esophagectomy complications consensus group (ECCG) proposed standardized definitions for complications after esophagectomy and hopefully this will lead to more uniform definitions of complications in future studies (22).
For open esophagectomy, the anastomotic leakage incidence has been shown to be higher after cervical anastomosis compared to intrathoracic anastomosis in a meta-analysis of randomized controlled trials (23). However, the included RCTs were of moderate methodological quality and included limited numbers of patients. None of the studies included patients undergoing MIE and this is important, since especially the minimally invasive creation of an intrathoracic anastomosis is considered to be technically challenging and results of open surgery might not be applicable to TMIE. HMIE might combine the best of both worlds for the Ivor Lewis procedure because the technically challenging thoracoscopic creation of an intrathoracic anastomosis is avoided by performing a thoracotomy and pulmonary complications are reduced by performing laparoscopic gastric mobilization (4).
Minimally invasive Ivor Lewis esophagectomy was associated with a lower incidence of RLN trauma and less blood loss. The difference in the incidence of RLN trauma is consistent with the literature regarding open procedures (23) and is explained by avoiding a cervical dissection close to the RLN. This is important, since it has been shown that RLN trauma is associated with increased incidence of pulmonary complications, postoperative ventilation time, intensive care length of stay and hospital length of stay (24-26). The lower blood loss volume that was found in patients undergoing minimally invasive Ivor Lewis esophagectomy can be explained by omitting a third stage, the incision and the associated blood loss.
An interesting finding is that the hospital length of stay was shorter after minimally invasive Ivor Lewis esophagectomy than after minimally invasive McKeown esophagectomy, despite the fact that no differences in postoperative complications were observed between the groups. This may be explained by the fact that patients with intrathoracic anastomosis have a lower incidence of functional morbidity. In addition to a lower RLN trauma incidence, fewer swallowing problems and a lower incidence of benign anastomotic dilatations (27) might contribute to a shorter hospital length of stay. Further, prospective research and comparisons of other groups are needed in order to assess whether this significant difference in functional results after intrathoracic anastomosis can be confirmed. Another explanation is that the lower length of stay in the Ivor Lewis group might have been caused by performance of the minimally invasive Ivor Lewis procedure in a more recent era, with increased surgeon experience, increased expertise in postoperative management, increased use of enhanced recovery after surgery protocols and improved intensive care. However, only one of the included studies described this phenomenon (19). Therefore, it remains unknown to what extent this selection bias has influenced the results of the included studies.
Taking into account the limitations of the current evidence, it remains uncertain whether a McKeown or an Ivor Lewis should be preferred for MIE for patients in which both procedures are oncologically feasible. To answer this question, the ICAN randomized controlled trial is currently being conducted in the Netherlands and this trial randomizes 200 patients between TMIE McKeown and TMIE Ivor Lewis. In addition to postoperative morbidity and the severity of complications, this trial is also powered for finding differences in quality of life, functional results and cost-effectiveness (28).
Strengths of this study are the comprehensive search strategy and the fact that this is the first review comparing TMIE McKeown versus TMIE Ivor Lewis. Limitations are the heterogeneity of the included studies regarding definitions of outcome parameters, the moderate methodological quality and the retrospective character of the included studies. In addition, selection bias might have played a significant role, but it is unclear to what extent it is present in the included studies. More research is needed in order to determine whether McKeown or Ivor Lewis MIE should be preferred for patients in whom both procedures are feasible.
Totally minimally invasive Ivor Lewis esophagectomy is associated with improved outcome regarding RLN trauma, hospital length of stay and blood loss compared to totally minimally invasive McKeown esophagectomy. However, the evidence is limited, of moderate quality and at risk for bias. A randomized controlled trial (Intrathoracic versus Cervical ANastomosis after transthoracic esophagectomy: ICAN trial) is currently being performed in order to demonstrate whether minimally invasive McKeown or minimally invasive Ivor Lewis esophagectomy should be preferred for patients in which both procedures are oncologically feasible.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Mariette M, Meunier B, Pezet D, et al. Hybrid minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicenter, open-label, randomized phase III controlled trial, the MIRO trial. J Clin Oncol 2015;33:suppl 3;abstr 5.
- Dantoc M, Cox MR, Eslick GD. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: a meta-analysis. Arch Surg 2012;147:768-76. [Crossref] [PubMed]
- Sihag S, Wright CD, Wain JC, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg 2012;42:430-7. [Crossref] [PubMed]
- Haverkamp L, Seesing MF, Ruurda JP, et al. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus 2017;30:1-7. [Crossref]
- Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg 1978;76:643-54. [PubMed]
- McKeown KC. Total three-stage oesophagectomy for cancer of the oesophagus. Br J Surg 1976;63:259-62. [Crossref] [PubMed]
- LEWIS I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg 1946;34:18-31. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [Crossref] [PubMed]
- Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.0.0. The Cochrane Collaboration. Available online: http://handbook.cochrane.org
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [Crossref] [PubMed]
- Nguyen NT, Hinojosa MW, Smith BR, et al. Minimally invasive esophagectomy: lessons learned from 104 operations. Ann Surg 2008;248:1081-91. [Crossref] [PubMed]
- Rajan PS, Vaithiswaran V, Rajapandian S, et al. Minimally invasive oesophagectomy for carcinoma oesophagus--approaches and options in a high volume tertiary centre. J Indian Med Assoc 2010;108:642-4. [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Hao Z, Zhenya S, Lei W. Esophageal-gastric anastomosis in radical resection of esophageal cancer under thoracoscopy combined with laparoscopy. J Coll Physicians Surg Pak 2014;24:754-6. [PubMed]
- Zhai C, Liu Y, Li W, et al. A comparison of short-term outcomes between Ivor Lewis and McKeown minimally invasive esophagectomy. J Thorac Dis 2015;7:2352-8. [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Biere SS, Maas KW, Cuesta MA, et al. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg 2011;28:29-35. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, Devriese PP, et al. Vocal cord paralysis after subtotal oesophagectomy. Br J Surg 1999;86:1583-7. [Crossref] [PubMed]
- Baba M, Natsugoe S, Shimada M, et al. Does hoarseness of voice from recurrent nerve paralysis after esophagectomy for carcinoma influence patient quality of life? J Am Coll Surg 1999;188:231-6. [Crossref] [PubMed]
- Hulscher JB, Tijssen JG, Obertop H, et al. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306-13. [Crossref] [PubMed]
- van Workum F, van der Maas J, van den Wildenberg FJ, et al. Improved Functional Results After Minimally Invasive Esophagectomy: Intrathoracic Versus Cervical Anastomosis. Ann Thorac Surg 2017;103:267-73. [Crossref] [PubMed]
- van Workum F, Bouwense SA, Luyer MD, et al. Intrathoracic versus Cervical ANastomosis after minimally invasive esophagectomy for esophageal cancer: study protocol of the ICAN randomized controlled trial. Trials 2016;17:505. [Crossref] [PubMed]