Disease flare after discontinuing gefitinib in a patient with lung adenocarcinoma and concomitant epithelial growth factor receptor mutation and anaplastic lymphoma kinase translocation
Introduction
During the past decade, the emerging effect of targeted therapies had led to a therapeutic paradigm shift in lung cancer (1). Personalized targeted therapy for non-small cell lung cancer (NSCLC) is based on mutation status of the epithelial growth factor receptor (EGFR) and translocation of anaplastic lymphoma kinase (ALK). EGFR tyrosine kinase inhibitors (TKIs) such as gefitinib, erlotinib or afatinib and ALK inhibitors such as crizotinib, ceritinib or alectinib have been shown significant benefit in advanced NSCLC patients harboring these driver mutations (2). However, almost all patients treated with TKIs against these driver mutations inevitably develop progressive disease because of acquired resistance after about a median of 12 months of disease control (3). An EGFR mutation and ALK translocation are generally considered mutually exclusive, and the coexistence of these two drivers has been reported in rare NSCLC cases and the effect on the response to targeted therapy is still unknown (4). Some studies have reported that these co-alterations are associated with a poor response to EGFR TKIs (5).
Stopping an EGFR-TKI can induce sudden and rapid disease exacerbation within a short time, which has been called a disease flare (6). Moreover, patients who develop these disease flares after stopping an EGFR-TKI have shorter post-TKI survival and poorer overall survival (7). Although patients with disease flare have shorter progression free survival to initial administration of a TKI than that of no-flare patients, little is known about the clinicopathological factors predicting the occurrence of a disease flare (6,8). Here, we show an interesting disease flare case after a patient with lung adenocarcinoma and a concomitant EGFR mutation and ALK translocation discontinued gefitinib.
Case presentation
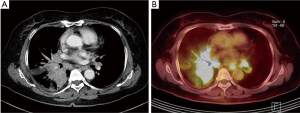
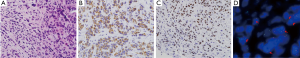
A 63-year-old woman was admitted to the hospital due to a productive cough for 2 months. A chest X-ray and a chest computed tomography (CT) scan revealed a central lung mass in the right middle and lower lobes with multiple lymph node metastases (T4N3) (Figure 1A). Positron emission tomography-CT revealed a hypermetabolic mass in the right lower lobe and uptake by multiple lymph nodes (Figure 1B). We conducted a tumor biopsy under bronchoscopy. The pathological diagnosis was poorly differentiated adenocarcinoma, and cytokeratin-7 and thyroid transcription factor-1 were positive on an immunohistochemistry marker assay (Figure 2A,B,C). EGFR genotyping was performed using a peptide nucleic acid-mediated polymerase chain reaction clamping method, and fluorescent in situ hybridization assay (FISH) was performed on tumor tissues using a break-apart probe specific to the ALK locus. We request scoring of an additional 50 tumor cells in the case of an ALK FISH split rate of 10–50% on the first 50 tumor cells. The final rate of rearranged-positive cells is calculated based on the sum of the first and second scores. According to the two-step assessment, ALK FISH showed 15.1% of tumor cells positive for ALK translocation (Figure 2D), a sensitive EGFR mutation (L858R mutation in exon 21) was identified.
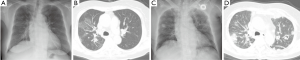
We decided to use gefitinib as first-line treatment but stable disease was achieved for only 4 months. We stopped the gefitinib due to disease progression, including growth of a mass and lymphangitic metastasis (Figure 3A,B) and treated her with pemetrexed. Crizotinib treatment was not covered with health insurance at that time of disease progression, and the patient refused to receive the crizotinib for that reason of the financial problem. The patient was admitted to the emergency room 22 days after stopping the gefitinib due to severe dyspnea and back pain. We found numerous hematogenous spreading nodules with cavitation and lymphangitic metastasis in both lungs on a chest X-ray and CT scan (Figure 3C,D) as well as extensive bone metastasis. Aggravation of respiratory failure and progression of multiple organ dysfunction led to death on admission day 4, which was 26 days after discontinuing the gefitinib.
Discussion
Patients with lung cancer who receive first-line TKI treatment for an EGFR mutation or ALK translocation have improved overall response and longer progression-free survival rates than those receiving standard chemotherapy (9,10). The concomitant occurrence of these two mutations is more frequent than expected, despite that they are conventionally considered mutually exclusive (11,12). Two EGFR/ALK co-mutant tumors demonstrated that genetic intratumoral heterogeneity coexists in both single-driver and EGFR/ALK co-adenocarcinoma and that mutant oncogenic drivers in spatially separate subclones of the same tumor might be different (13). However, EGFR/ALK co-mutant patients show diverse responses to EGFR-TKIs and crizotinib, and some cases respond poorly to EGFR-TKI (14).
Disease flare is defined as hospitalization or death attributable to tumor progression after stopping a TKI and before initiating subsequent therapy. The incidence of a disease flare in patients with EGFR-mutant lung cancer is 4–23%, and median time to disease flare after stopping a TKI is about 8 days (6-8). The disease flare mechanism is not yet fully understood. We suggest that when the EGFR-TKI is stopped in patients with EGFR activating mutations, cell growth is accelerated in the sensitive clones, resulting in rapid and symptomatic progression (15). Although disease flares have not been associated with EGFR mutation status, a specific TKI, age, sex, or tobacco use, patients with a shorter response to initial TKI administration and pre-existing brain or pleural metastasis tend to have disease flares after stooping the TKI (6,7).
In this patient, discontinuing gefitinib led to fatal multi-organ failure due to hematogenous lung metastasis and extensive bone metastasis, which was compatible with the previously reported poor prognosis (7). Pleura, lung, brain, and bone are known progressive sites in patients with a disease flare (6). EGFR TKI rechallenge or ALK inhibitors could be one of therapeutic options for disease flare in this patient. However, it was not available due to the financial problem. We selected the pemetrexed as a second-line therapy as pemetrexed has been known to be an effective option for patients with NSCLC harboring sensitive EGFR mutation after gefitinib failure in retrospective studies (16,17).
The ALK translocation is detected by FISH based on the first clear cut-off criterion (≥15% of tumor cells) for split or single red signals (18). Our FISH results met the criteria of at least 50 cells counted and at least 15% of the counted cells, as described previously (12). We hypothesized that the co-altered ALK translocation might influence short progression free survival compared with the average response to an EGFR-TKI. Additionally, despite the intratumoral heterogeneity observed with the EGFR/ALK co-mutation, the patient’s EGFR pathway had strong oncogene-addiction, which led to rapid deterioration and poor clinical outcome.
In conclusion, we experienced a rare case of disease flare after stopping gefitinib in a patient with lung adenocarcinoma and a concomitant activating EGFR mutation and ALK translocation. A disease flare should be considered after stopping an EGFR-TKI in patients with the EGFR/ALK co-mutation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: This manuscript was approved by the Institutional Review Board of Uijeongbu St. Mary’s Hospital, which waived the requirement from informed consent.
References
- Reck M, Heigener DF, Mok T, et al. Management of non-small-cell lung cancer: recent developments. Lancet 2013;382:709-19. [Crossref] [PubMed]
- Rocco G, Morabito A, Leone A, et al. Management of non-small cell lung cancer in the era of personalized medicine. Int J Biochem Cell Biol 2016;78:173-9. [Crossref] [PubMed]
- Ohashi K, Maruvka YE, Michor F, et al. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol 2013;31:1070-80. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Zhou J, Zheng J, Zhao J, et al. Poor response to gefitinib in lung adenocarcinoma with concomitant epidermal growth factor receptor mutation and anaplastic lymphoma kinase rearrangement. Thorac Cancer 2015;6:216-9. [Crossref] [PubMed]
- Chaft JE, Oxnard GR, Sima CS, et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res 2011;17:6298-303. [Crossref] [PubMed]
- Chen HJ, Yan HH, Yang JJ, et al. Disease flare after EGFR tyrosine kinase inhibitor cessation predicts poor survival in patients with non-small cell lung cancer. Pathol Oncol Res 2013;19:833-8. [Crossref] [PubMed]
- Akamatsu H, Ono A, Shukuya T, et al. Disease flare after gefitinib discontinuation. Respir Investig 2015;53:68-72. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N Engl J Med 2015;373:1582.
- Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res 2013;19:4273-81. [Crossref] [PubMed]
- Kim TJ, Park CK, Yeo CD, et al. Simultaneous diagnostic platform of genotyping EGFR, KRAS, and ALK in 510 korean patients with Non-Small-Cell lung cancer highlights significantly higher ALK rearrangement rate in advanced stage. J Surg Oncol 2014;110:245-51. [Crossref] [PubMed]
- Cai W, Lin D, Wu C, et al. Intratumoral heterogeneity of ALK-Rearranged and ALK/EGFR coaltered lung adenocarcinoma. J Clin Oncol 2015;33:3701-9. [Crossref] [PubMed]
- Yang JJ, Zhang XC, Su J, et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res 2014;20:1383-92. [Crossref] [PubMed]
- Chmielecki J, Foo J, Oxnard GR, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med 2011;3:90ra59. [Crossref] [PubMed]
- Yang CJ, Tsai MJ, Hung JY, et al. Pemetrexed had significantly better clinical efficacy in patients with stage IV lung adenocarcinoma with susceptible EGFR mutations receiving platinum-based chemotherapy after developing resistance to the first-line gefitinib treatment. Onco Targets Ther 2016;9:1579-87. [Crossref] [PubMed]
- Park S, Keam B, Kim SH, et al. Pemetrexed singlet versus Nonpemetrexed-Based Platinum doublet as Second-Line chemotherapy after First-Line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor failure in non-small cell lung cancer patients with EGFR mutations. Cancer Res Treat 2015;47:630-7. [Crossref] [PubMed]
- Thunnissen E, Bubendorf L, Dietel M, et al. EML4-ALK testing in non-small cell carcinomas of the lung: a review with recommendations. Virchows Arch 2012;461:245-57. [Crossref] [PubMed]


