Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis
Introduction
Traditionally, the management of patients with stage I non-small cell lung cancer (NSCLC) includes lobectomy associated to complete lymph node dissection through a conventional thoracotomy or thoracoscopic approach. Conversely, sublobar resections are considered the treatment of choice in patients with a compromised cardiorespiratory status (1).
In recent years, along with the line of what already happened in breast surgery, many factors have revamped the interest in sublobar resections, segmentectomies in particular, as preferred procedures for early lung cancers. In fact, with the technical advances in thoracic imaging and the use of low-dose computed tomography in screening programs as well as more accurate patient selection strategies, thoracic surgeons will likely encounter a significant increase in the number of small peripheral lesions in clinical practice (2).
The main advantage of the segmentectomy over lobectomy is that it is an anatomical resection with a parenchyma sparing-effect. However, whether anatomic segmentectomy is comparable with lobectomy about oncologic outcomes in patients with stage I disease is still debated in the medical and surgical community. The aim of this systematic review and meta-analysis is to compare the results of segmentectomy versus lobectomy regarding overall survival (OS) in the surgical treatment of early stage (stage I) NSCLC, as stated in the conclusions of the largest studies conducted in this field and reported and reported to date.
Methods
Search strategy and selection criteria
A search strategy using a combination of free-text words, relevant MeSH terms and appropriate filters was designed; the searching strategy was developed in EMBASE (via Ovid), MEDLINE (via PubMed) and Cochrane CENTRAL from 1990 until December 2016, without imposing any language or time restrictions (see section “Search history” in Supplementary). Records identified through our search strategy were imported into reference management software. The eligibility criteria were: stage I NSCLC patients; segmentectomy without wedge resection; comparison of recurrence-free survival, OS between lobectomy and segmentectomy. Two authors worked independently to assess each identified study based on the eligibility criteria; when multiple studies contained overlapping data, a most informative study was included. Letters, editorials, case reports, and reviews were excluded. Disagreements were discussed and resolved by consensus. Data extracted included study characteristics, baseline patient characteristics primary and secondary outcomes. We selected papers in the meta-analysis that also included wedge resections, but only when the OS was distinguished in the two groups of surgical techniques of sublobar resection (wedge and segmentectomy). The Cochrane’s Collaboration Tool was used to assess the risk of bias for the primary outcome for included studies (3). The risk of bias due to incomplete outcome data was evaluated at an outcome level, while the risk of bias due to sequence generation, allocation concealment, blinding, selective reporting or funding was assessed at study level. The risk of bias was assessed by two independent reviewers and disagreements were settled by discussion and consensus [see section “Risk of bias assessment of the primary outcome (immediate success)” in Supplementary]. Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at http://www.crd.york.ac.uk/prospero/display_record.asp?ID=CRD42016040153. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement was used to improve the report of this systematic review (Table S1) (4).

Full table
Data analysis
The meta-analysis was performed by combining the reported survival outcomes of the individual studies using a random effect model. The OS of the lobectomy group was compared with the segmentectomy group alone. The hazard ratio (HR) and standard error were extracted or calculated from each study using Kaplan-Meier graphs with methods reported in the literature (5,6). Confidence intervals (CI) were set to 95%. Heterogeneity was measured using χ2 test and I2. Values of P<0.10 or I2>50% represented substantial heterogeneity. The publication bias was assessed for the primary outcome with a Funnel plot, both visually and formally with Egger’s test (P<0.10 suggests strong asymmetry). Data analysis was performed using Review Manager 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark).
Role of the funding source
There was no source of financing for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
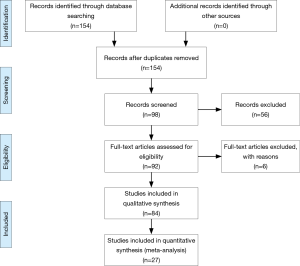
The flow diagram of the study selection process was shown in Figure 1. The search strategy identified 154 records. Following deduplication, 98 records were screened at the title and abstract level, and six were excluded as irrelevant. The remaining 92 records were assessed in the full text. Of those, 27 were included in the systematic review (7-21) and meta-analysis (22-33).
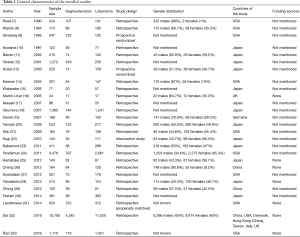
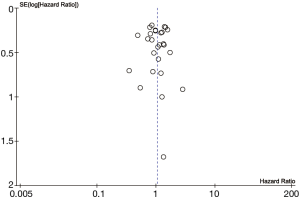
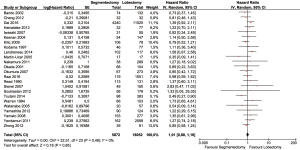
Baseline characteristics of patients were well balanced in each study. Study characteristics are presented in Table 1 and the quality assessment in Table 2. Most of these qualified studies were based on the retrospective data. The size of the cohorts varied from 17 to 11,520, with a total number of 24,542 patients. In all HR calculations, the lobectomy was chosen as the reference. None of the included trials was blinded. However, lack of blinding was not considered likely to influence the primary outcome due to its objective nature. Hence, all studies were at low risk of bias despite being open label. Similarly, the risk of bias was low for all other domains. Hence, all trials were at low overall risk of bias. The pooled HR was 1.04 (95% CI, 0.92–1.18; P=0.50). The segmentectomy group was not inferior to patients treated with lobectomy. The Cochrane tests for heterogeneity showed that χ2=25.04 degree of freedom =26 (P=0.52); I2=0%, which did not suggest a significant inconsistency and heterogeneity between the selected studies. The combined HR displayed in this figure suggested there was no statistical significance between segmentectomy and lobectomy on OS (Figure 2). The funnel plot showed no publication bias (Figure 3).
Full table

Full table
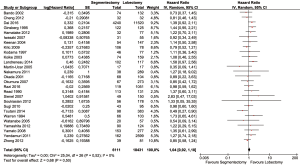
The whole group of papers was then divided in two subgroups regarding the methodology of the study: the first group comprehends the observational studies (24/27), the second the randomized studies (3/27). The HR for retrospective study (HR =1.01; 95% CI, 0.88–1.16, P=0.49) and for randomized clinical trial were not both significant (HR =1.21; 95% CI, 0.89–1.63, P=0.48) (Figures 4 and 5).

Discussion
The refinement of the diagnostic pathway for NSCLC and the recent consolidation of minimally invasive techniques for lobectomy and segmentectomy have revived the debate about whether segmentectomies should be applied to all patients with early stage NSCLC as intentional resection and not only as compromised procedure in patients with limited cardiopulmonary reserves. To better understand what has been published in the literature until today and to have a current state of the art, we combined data from 26 studies published from 1990 to 2016 and performed a meta-analysis by combining the OS in the lobectomy and segmentectomy groups for patients with stage I NSCLC. Overall, the patients in the lobectomy group did not have a better survival than the patients treated with segmentectomy. In particular, a significant benefit of lobectomy over segmentectomy on OS in patients with stage IA disease could not be confirmed.
Other meta-analyses also support these findings. As an example, Cao et al. stated that patients intentionally selected for segmentectomies to treat early-stage, peripheral NSCLC had overall disease-free survival outcomes that were not significantly different to those undergoing lobectomies. Conversely, patients who underwent “compromise” segmentectomies due to medical comorbidities or cardiopulmonary limitations showed a significantly worse OS than lobectomies (34).
Other published meta-analysis suggested that segmentectomy may not have superior oncologic outcomes for all patients with early stage lung cancer, but only for the subgroups of patients with tumors smaller than 2 cm (35) or for patients in stage IA (36).
This study presents some limitations. An ideal meta-analysis should be performed using individual patient data, but they may not always be available or practical. Therefore, the majority of meta-analyses are performed using summary data, which is a well-accepted form of analysis.
This study did not include any data about additional chemotherapy or radiotherapy regimens, which might have affected the survival of some patients. Also, since most of the retrospective studies did not describe with accuracy whether the stage was clinical or pathological, it is reasonable to conclude that the authors of the papers subjected to meta-analysis have selected the surgical method mainly based on the clinical stage. Similarly, the comparison between procedures in patients who can tolerate the lobectomy should be more compelling. In fact, most studies did not take into consideration systematic, or sampling lymphadenectomy is emphasising the current technical differences in lymph node management and anatomical approach. In this setting, segmentectomies are more often accompanied by hilar and mediastinal lymph node sampling than dissection (34).
Another potentially critical issue as a source of bias results from the fact that many studies comparing segmentectomy and lobectomy for stage IA NSCLC did not take into consideration the appearance of the nodule on chest CT, i.e., pure solid, part-solid or pure ground-glass opacity (GGO).
The current meta-analysis disclosed that segmentectomies produce similar survival compared to lobectomy for patients with stage I NSCLC. Considering heterogeneity among studies and most of the data from retrospective studies, the results of the meta-analysis should be interpreted with caution.
More evidence is needed to establish what is the role of segmentectomy in early NSCLC, in particular, a large numbered, prospective, randomised trials, which should dissolve the uncertainties and the questions raised by retrospective data. Otherwise, propensity score matching method is required in a retrospective study, since the patients’ selection bias could be incredibly high in such data accumulation.
Currently, two prospective, randomised, multi-institutional phase III trials are being conducted by the Cancer and Leukemia Group B (CALGB 140503) and the Japan Clinical Oncology Group (JCOG 0802) to establish the effectiveness of intentional sublobar resections for small peripheral tumours (2 cm) (37-39). The results will likely provide significant contributions to the role of intentional resection for peripheral stage IA tumours.
In conclusion, our analysis seems to support the concept that rigorously selected patients with early-stage NSCLC may be subjected to segmentectomies rather than lobectomies with similar survival outcomes and the benefit of preserving lung function.
Search history
- Medline; exp LUNG NEOPLASMS/SU [SU=surgery]; 180503 results.
- Medline; (segmentectom* OR “limit* resect*” OR sublobar).ti,ab; 3516 results.
- Medline; (intention* OR compromis*).ti,ab; 146950 results.
- Medline; 1 AND 2 AND 3; 72 results.
- Medline; (lung OR pulmo*).ti,ab; 749424 results.
- Medline; 2 AND 3 AND 5; 86 results.
- Medline; 6 [Limit to: (Document Status In Data Review or In Process)]; 2 results.
- EMBASE; exp LUNG TUMOR/su [su=Surgery]; 22940 results.
- EMBASE; (segmentectom* OR “limit* resect*” OR sublobar).ti,ab; 4856 results.
- EMBASE; (intention* OR compromis*).ti,ab; 188421 results.
- EMBASE; 8 AND 9 AND 10; 52 results.
Risk of bias assessment of the primary outcome (immediate success)
We assessed the risk of bias utilising the Cochrane Collaboration risk of bias tool for sequence generation, allocation concealment, blinding, selective reporting, incomplete outcome data and other sources of bias (sponsorship bias). The risk of bias for sequence generation, allocation concealment, blinding, selective outcome reporting and sponsor bias were assessed at study level. The risk of bias for incomplete outcome data was evaluated at outcome level. We then determined the overall risk of bias.
Sequence generation
- Low risk of bias, if randomization was generated by a computer or a table of random numbers.
- High risk of bias, if the method of randomization was inadequate (i.e. “quasi-randomized”).
- Unclear risk of bias, if the method of randomization was not described.
Allocation concealment
- Low risk of bias, if the method of allocation involved an independent central unit or consecutively numbered sealed envelopes.
- High risk of bias, if allocation sequence was known to the investigators or conducted with an inadequate method.
- Unclear risk of bias, if the method of allocation concealment was not described.
Blinding of participants and personnel
- Low risk of bias if the study had a double-blind design and unlikely that the blinding could have been broken, or if no blinding or incomplete blinding but the review authors judge that the outcome is not likely to be influenced by the lack of blinding.
- High risk of bias, if the study was open-label and the outcome is likely to be affected by the lack of blinding, or if blinding of the main study participants and personnel attempted but likely that the blinding could have been broken, and the outcome is likely to be influenced by the lack of blinding.
- Unclear risk of bias, if there was insufficient information to permit judgment of ‘Low risk’ or ‘High risk’.
Selective outcome reporting
- Low risk of bias if the trial provided data about immediate success and recurrence for the follow-up period.
- High risk of bias if data about instant success and recurrence for the given follow-up period were reported with inadequate detail for the data to be included in the meta-analysis or if it was reported only for a subset of the randomised population.
- Unclear risk of bias, if there was insufficient information to assess whether the risk of bias of selective outcome reporting was present.
Incomplete outcome data
- Low risk of bias, if attrition rate was balanced between treatment arms and relatively low (below 20%), reasons for discontinuation were described, an intention-to-treat analysis was performed and an appropriate method of imputation of missing outcome data was applied.
- High risk of bias, if withdrawal rates were unbalanced between treatment arms or more than 20%, or reasons for drop-outs were not clearly described, or an inappropriate analysis was performed (i.e. per protocol analysis), or an inappropriate imputation method (i.e. last observation carried forward method) was used to handle missing data.
- Unclear risk of bias, if it was not clear whether there were any drop-outs or reasons for these withdrawals were not clear, or no method of imputation of missing data was mentioned.
Other bias (sponsor bias)
- Low risk of bias, if the trial did not receive commercial funding.
- High risk of bias, if the trial received commercial funding.
- Unclear risk of bias, if the source of funding was unclear.
Overall risk of bias
- The overall risk was considered high in the presence of high bias in any domain, or low when low for all domains.
- In all other cases, the overall risk of bias was deemed unclear.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Sihoe AD, Van Schil P. Non-small cell lung cancer: when to offer sublobar resection. Lung Cancer 2014;86:115-20. [Crossref] [PubMed]
- Higgins JP, Green S. editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Updated March 2011. The Cochrane Collaboration, 2011. Available online: http://www.cochrane-handbook.org
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [Crossref] [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [Crossref] [PubMed]
- Guyot P, Ades A, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol 2012;12:9. [Crossref] [PubMed]
- Read RC, Yoder G, Schaeffer RC. Survival after conservative resection for T1 N0 M0 non-small cell lung cancer. Ann Thorac Surg 1990;49:391-8; discussion 399-400. [Crossref] [PubMed]
- Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 1994;107:1087-93; discussion 1093-4. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Kodama K, Doi O, Higashiyama M, et al. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg 1997;114:347-53. [Crossref] [PubMed]
- Bando T, Yamagihara K, Ohtake Y, et al. A new method of segmental resection for primary lung cancer: intermediate results. Eur J Cardiothorac Surg 2002;21:894-9; discussion 900. [Crossref] [PubMed]
- Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60; discussion 961. [Crossref] [PubMed]
- Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg 2003;125:924-8. [Crossref] [PubMed]
- Keenan RJ, Landreneau RJ, Maley RH Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. [Crossref] [PubMed]
- Watanabe T, Okada A, Imakiire T, et al. Intentional limited resection for small peripheral lung cancer based on intraoperative pathologic exploration. Jpn J Thorac Cardiovasc Surg 2005;53:29-35. [Crossref] [PubMed]
- Martin-Ucar AE, Nakas A, Pilling JE, et al. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg 2005;27:675-9. [Crossref] [PubMed]
- Iwasaki A, Hamanaka W, Hamada T, et al. Comparison between a case-matched analysis of left upper lobe trisegmentectomy and left upper lobectomy for small size lung cancer located in the upper division. Thorac Cardiovasc Surg 2007;55:454-7. [Crossref] [PubMed]
- Okumura M, Goto M, Ideguchi K, et al. Factors associated with outcome of segmentectomy for non-small cell lung cancer: long-term follow-up study at a single institution in Japan. Lung Cancer 2007;58:231-7. [Crossref] [PubMed]
- Sienel W, Stremmel C, Kirschbaum A, et al. Frequency of local recurrence following segmentectomy of stage IA nonsmall cell lung cancer is influenced by segment localisation and width of resection margins-implications for patientselection for segmentectomy. Eur J Cardiothorac Surg 2007;31:522-7; discussion 527-8. [Crossref] [PubMed]
- Yamato Y, Koike T, Yoshiya K, et al. Results of surgical treatment for small (2 cm or under) adenocarcinomas of the lung. Surg Today 2008;38:109-14. [Crossref] [PubMed]
- Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg 2009;87:1662-6; discussion 1667-8.
- Sugi K, Kobayashi S, Sudou M, et al. Long-term prognosis of video-assisted limited surgery for early lung cancer. Eur J Cardiothorac Surg 2010;37:456-60. [PubMed]
- Nakamura H, Taniguchi Y, Miwa K, et al. Comparison of the surgical outcomes of thoracoscopic lobectomy, segmentectomy, and wedge resection for clinical stage I non-small cell lung cancer. Thorac Cardiovasc Surg 2011;59:137-41. [Crossref] [PubMed]
- Yendamuri S, Gold D, Jayaprakash V, et al. Is sublobar resection sufficient for carcinoid tumors? Ann Thor Surg 2011;92:1774-8; discussion 1778-9.
- Hamatake D, Yoshida Y, Miyahara S, et al. Surgical outcomes of lung cancer measuring less than 1 cm in diameter. Interact Cardiovasc Thorac Surg 2012;15:854-8. [Crossref] [PubMed]
- Cheng YD, Duan CJ, Dong S, et al. Clinical controlled comparison between lobectomy and segmental resection for patients over 70 years of age with clinical stage I non-small cell lung cancer. Eur J Surg Oncol 2012;38:1149-55. [Crossref] [PubMed]
- Soukiasian HJ, Hong E, McKenna RJ Jr. Video-assisted thoracoscopic trisegmentectomy and left upper lobectomy provide equivalent survivals for stage IA and IB lung cancer. J Thorac Cardiovasc Surg 2012;144:S23-6. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8. [Crossref] [PubMed]
- Zhong C, Fang W, Mao T, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg 2012;94:362-7. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Sublobar resection for lung adenocarcinoma meeting node-negative criteria on preoperative imaging. Ann Thorac Surg 2014;97:1701-7. [Crossref] [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [Crossref] [PubMed]
- Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients with Non Small-Cell Lung Cancer ≤ 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016;34:3175-82. [Crossref] [PubMed]
- Razi SS, John MM, Sainathan S, et al. Sublobar resection is equivalent to lobectomy for T1a non-small cell lung cancer in the elderly: a Surveillance, Epidemiology, and End Results database analysis. J Surg Res 2016;200:683-9. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?—A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Bao F, Ye P, Yang Y, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis. Eur J Cardiothorac Surg 2014;46:1-7. [Crossref] [PubMed]
- Zhang L, Li M, Yin R, et al. Comparison of the oncologic outcomes of anatomic segmentectomy and lobectomy for early-stage non-small cell lung cancer. Ann Thorac Surg 2015;99:728-37. [Crossref] [PubMed]
- Altorki N, Kohman LJ, Veit LJ, et al. Limited resection as a cure for early lung cancer: time to challenge the gold standard? Bull Am Coll Surg 2015;100:57-8. [PubMed]
- Blasberg JD, Pass HI, Donington JS. Sublobar Resection: A Movement from the Lung Cancer Study Group. J Thorac Oncol 2010;5:1583-93. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized tial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]


