Rapid adoption of transcatheter aortic valve replacement in intermediate- and high-risk patients to treat severe aortic valve stenosis
The field of transcatheter aortic valve replacement (TAVR) is rapidly evolving, with major refinements in technology, procedural techniques, and patient selection. Initially, TAVR was shown to improve survival in inoperable patients compared with medical treatment or balloon valvuloplasty (1) and to be non-inferior to surgical aortic valve replacement (SAVR) in high-risk patients with severe, symptomatic aortic valve stenosis (2,3). In the meta-analysis by Carnero-Alcázar et al., early and late outcomes and hemodynamic performance of TAVR versus SAVR in intermediate to high-risk patients are compared (4).
Based on pooled data from more than 10,000 patients, Carnero-Alcázar et al. demonstrate that early and late mortality are similar with TAVR and SAVR in intermediate and high-risk patients (4). This is in alignment with another meta-analysis including 3,806 participants who are randomly assigned to undergo TAVR or SAVR, showing that the transcatheter approach was associated with a significant 13% reduction in all-cause mortality after 2 years of follow-up (5).
In the large randomized controlled trials, TAVR has indeed been shown to be non-inferior or even superior to SAVR with respect to all-cause mortality in patients at high surgical risk (2,3). In patients at intermediate risk, TAVR has been reported non-inferior to SAVR regarding death from any cause or disabling stroke—as recently published in the New England Journal of Medicine in two independent clinical trials [PARTNER-2 (6) and SURTAVI (7)]. In the transfemoral cohort of the PARTNER-2 trial, TAVR even resulted in a lower rate of death or disabling stroke than surgery (6). In addition, the first randomized trial comparing TAVR and SAVR in all-comer patients (NOTION) indicated that these findings also apply to patients at even lower surgical risk (8). Figure 1 gives an overview of all-cause mortality rates reported for the different randomized controlled trials comparing TAVR and SAVR (6-12). Clearly, long-term follow-up data are needed for the lower risk patient populations; however, it will just be a matter of time before these data will become available (clinicaltrials.gov: NCT02825134—NOTION-2, NCT02675114—PARTNER-3, NCT02701283—TAVR-low risk).
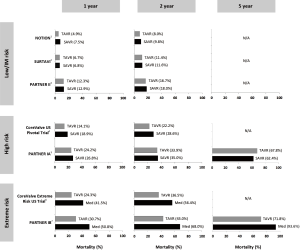
Concerning early or long-term stroke risk, the pooled analysis by Carnero-Alcázar et al. representing 15,375 patients demonstrated no statistically significant difference among patients assigned to TAVR versus SAVR (4). In order to further reduce the risk of peri-procedural stroke in TAVR, several cerebral protection devices are currently under investigation. Although the use of a cerebral protection device reduced the frequency of ischemic cerebral lesions in the CLEAN-TAVI randomized clinical trial (13), larger studies are needed to assess the effect of cerebral protection device use on neurological and cognitive function after TAVR. Another important observation in this context is the possible association of subclinical leaflet thrombosis and increased rates of neurological events (14,15). Subclinical leaflet thrombosis has recently been reported to occur frequently in bioprosthetic aortic valves, more commonly in transcatheter than in surgical valves (15). Anticoagulation, but not dual antiplatelet therapy, is effective in prevention or treatment of subclinical leaflet thrombosis (14,15). Despite excellent outcomes after TAVR with the new-generation valves, prevention and treatment of subclinical leaflet thrombosis might offer a potential opportunity for further improvement in clinical outcomes. Two ongoing randomized controlled trials are specifically investigating which anti-thrombotic strategy might be best following TAVR (clinicaltrials.gov: NCT02556203—GALILEO, NCT02664649—ATLANTIS). Finally, another important observation is that most studies report a lower rate of new-onset atrial fibrillation in the TAVR group as compared to the SAVR group (6-12); whether this may be associated with a lower stroke risk at medium term follow-up for TAVR patients still has to be determined. In addition, a number of other procedural complications such as acute kidney injury and major bleeding are only half as common after TAVR than SAVR (4). However, to justify expansion of TAVR into low-risk patients who can undergo SAVR with excellent outcome, the transcatheter technology needs to address some of its own initial shortcomings.
Moderate or severe paravalvular leakage (PVL) was initially reported in 10% to 15% of patients treated with TAVR (1,2) and has been associated with increased mortality. However, more accurate sizing of the aortic annulus based on cardiac computed tomography (CT) imaging—instead of transesophageal echocardiography (TEE) imaging—resulted in better PVL outcomes over the past few years. In addition, newer generation transcatheter heart valves have an outer skirt or adaptive seal, and some systems are even retrievable to optimize the implantation position. These improvements have resulted in low single-digit moderate-to-severe PVL rates in the latest TAVR studies (3,16,17).
Due to the proximity between the transcatheter valve frame extending into the left ventricular outflow tract and the conduction system, heart block with need for permanent pacemaker implantation has been frequent after TAVR. Although new permanent pacemaker implantation adds to the risk of procedural complications and overall cost, it protects against unexpected death, probably due to the inherent risk of complete heart block among patients with severe aortic stenosis (18). Longer-term follow-up studies will have to investigate the impact of this permanent pacemaker implantation/use on left ventricular function, risk for device-related infection, and quality of life. The appreciation of the importance of higher prostheses implantation, as well as introduction of re-positional TAVR systems have lowered the need for permanent pacemaker to 10% to 15% for most systems (4).
Recently, some concern was raised about potential poor long-term durability of TAVR bioprostheses. Combined data from early adopting TAVR centres in Rouen and Vancouver presented by Dvir et al. at EuroPCR 2016 suggested relatively high rates of structural degeneration of first-generation TAVR devices implanted 5 to 14 years ago, particularly in subjects with renal failure (19). Amongst 378 high-risk elderly patients with a median survival time of 51 months, structural valve degeneration—defined as at least moderate aortic regurgitation AND/OR mean gradient >20 mmHg which was not present within 30 days of the index procedure—was present in 35 subjects (9.3%). However, these preliminary data were criticized because of several methodological concerns. First of all, only echocardiographic findings were used to define valve degeneration, which is in contrast with the “need for re-intervention” used as definition for surgical valve degeneration. Another important limitation was the small number of subjects still at risk beyond 5 years (n<100), causing a failure to appreciate the hazards at long-term in a reliable way (20). Clearly, the only way to get reliable long-term durability data is to introduce the therapy into younger patients, preferably in randomized clinical trials against SAVR. Importantly, robust long-term follow-up data comparing TAVR and SAVR do not reveal any difference in valve performance and durability at present—and this with even 5-year echocardiographic follow-up data (10,12).
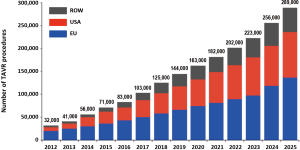
Finally, it will be important to investigate the impact of TAVR versus SAVR on patient satisfaction and health economics in future studies. Due to the ageing overall population in Europe, North America and Asia, we should be prepared for an exponentially growing demand for aortic valve replacement within the next decade(s) (Figure 2) (21). The TAVR technology—with its ability to replace a diseased aortic valve in a true minimalistic approach and with a minimum of hospitalization length—could be the ideal technology to answer this demand.
In conclusion, the rapid expansion of TAVR has been based upon robust clinical evidence derived from randomized controlled trials and large-scale national and international registries—in many nations, the volume of TAVR now exceeds SAVR. Trials in younger and low-risk patients are ongoing. However, continued follow-up of existing research populations as well as further study of the TAVR technology in challenging conditions—such as bicuspid aortic valves, pure native aortic valve regurgitation, valve-in-valve, etc.—will be needed to further establish the TAVR technology as the default treatment option for most patients with severe aortic stenosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Adams DH, Popma JJ, Reardon MJ, et al. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. [Crossref] [PubMed]
- Carnero-Alcázar M, Maroto LC, Cobiella-Carnicer J, et al. Transcatheter versus surgical aortic valve replacement in moderate and high-risk patients: a meta-analysis. Eur J Cardiothorac Surg 2017;51:644-52. [PubMed]
- Siontis GC, Praz F, Pilgrim T, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: a meta-analysis of randomized trials. Eur Heart J 2016;37:3503-12. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321-31. [Crossref] [PubMed]
- Thyregod HG, Steinbrüchel DA, Ihlemann N, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J Am Coll Cardiol 2015;65:2184-94. [Crossref] [PubMed]
- Reardon MJ, Adams DH, Kleiman NS, et al. 2-Year Outcomes in Patients Undergoing Surgical or Self-Expanding Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2015;66:113-21. [Crossref] [PubMed]
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014;63:1972-81. [Crossref] [PubMed]
- Kapadia SR, Leon MB, Makkar RR, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2485-91. [Crossref] [PubMed]
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a Cerebral Protection Device on Brain Lesions Following Transcatheter Aortic Valve Implantation in Patients With Severe Aortic Stenosis: The CLEAN-TAVI Randomized Clinical Trial. JAMA 2016;316:592-601. [Crossref] [PubMed]
- Makkar RR, Fontana G, Jilaihawi H, et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N Engl J Med 2015;373:2015-24. [Crossref] [PubMed]
- Chakravarty T, Søndergaard L, Friedman J, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Kodali S, Thourani VH, White J, et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J 2016;37:2252-62. [Crossref] [PubMed]
- Meredith Am IT, Walters DL, Dumonteil N, et al. Transcatheter aortic valve replacement for severe symptomatic aortic stenosis using a repositionable valve system: 30-day primary endpoint results from the REPRISE II study. J Am Coll Cardiol 2014;64:1339-48. [Crossref] [PubMed]
- Urena M, Webb JG, Tamburino C, et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation 2014;129:1233-43. [Crossref] [PubMed]
- Dvir D. First look at long-term durability of transcatheter heart valves: assessment of valve function up to 10 years after implantation. Available online: http://www.crtonline.org/presentation-detail/first-look-at-long-term-durability-of-transcathete
- Prendergast B, Tamburino C, Piazza N, et al. Durability of transcatheter heart valves —much ado about nothing. EuroIntervention 2016;12:819-20. [Crossref] [PubMed]
- Credit Suisse TAVI Comment, 2015. ASP assumption for 2025 based on analyst model. Available online: https://image.slidesharecdn.com/r2coptimization-160427203509/95/r2-c-optimization-18-638.jpg?cb=1461789345


