Combined analgesic treatment of epidural and paravertebral block after thoracic surgery
Introduction
Thoracotomy induces severe postoperative pain, which can cause respiratory complications such as hypoxia, atelectasis and pulmonary infections (1). In addition, inadequate pain control can lead to post-thoracotomy pain syndrome, which may continue for many years (2), thus an appropriate pain management is essential after surgery. Conventionally, the most common pain management after chest surgery is epidural analgesia (EPI). EPI is clearly effective in managing the pain, however, it still has contraindications and the risk of severe complications. In addition, the reported failure rates of EPI are as high as 12% (3), and the effects of this analgesia vary among patients. Recently, paravertebral block (PVB) has been reported as an alternative to EPI (4-7). PVB has equivalent analgesic effects to EPI and have a lower incidence of side effects, such as nausea, vomiting, hypotension and urinary retention (8-10).
Although many comparative studies of EPI versus PVB have been reported, the synergistic effects of EPI and PVB have not yet been investigated. In our institution, we applied the combination method of EPI and PVB as a management of thoracotomy pain since November 2014 to prevent analgesic failures and with the expectant that it can have the superior analgesic effects to single method of EPI or PVB. In this study, we established PVB catheter insertion using a metal suction tube and evaluated the safety and feasibility of the combination of EPI and PVB.
Operative techniques
This study included patients who underwent thoracic surgery for pulmonary, mediastinal and pleural lesions and were subject to our novel analgesic treatment of combined EPI and PVB blocks between November 2014 and September 2016. The study was approved by the institutional ethics board of Yamanashi Central Hospital. The patients provided written informed consent for the studies.
From November 2014 to December 2016, 31 patients received oral anti-coagulant or anti-platelet agents before surgery, of whom 13 were unable to receive EPI and received only PVB. An additional 61 patients received only EPI, as the use of surgical procedures such as thoracic wall resection, median sternotomy, and anterior thoracotomy precluded the use of PVB. Only three patients were unable to receive either EPI or PVB. This study therefore included 368 patients who received the combination analgesic treatment of EPI and PVB after thoracotomy (Table 1). Average age was 61.6 years (range, 15–88 years). Of all the patients, 227 patients were male, 141 were female (Table 1); 281 patients underwent standard thoracotomy, 26 patients underwent small incision thoracotomy, 61 patients underwent video-assisted thoracoscopic surgery (VATS) (Table 1). The incision length of standard thoracotomy was approximately 10 cm, and that of small incision thoracotomy was approximately 5 cm. Both were placed over 5th intercostal space. Our VATS approach involved three incisions, each were approximately 1.2 cm. Two hundred-fifty-three cases underwent lobectomy, 94 wedge resection, 6 segmentectomy, 6 tumorectomy for mediastinal or pleural tumors, 4 pneumonectomy, 2 lymph node biopsy, 2 pulmorrhaphy, and 1 chest wall resection (Table 1). Lung cancer was diagnosed in 261 cases, pneumothorax in 83, metastatic cancer in 8, mediastinal tumor in 6, inflammatory lesion in 4, lymphoma in 3, benign lung tumor in 2 and chest wall tumor in 1 case (Table 1).
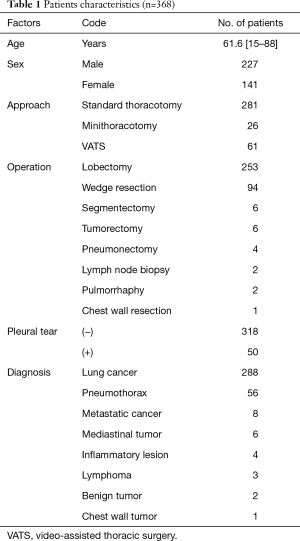
Full table
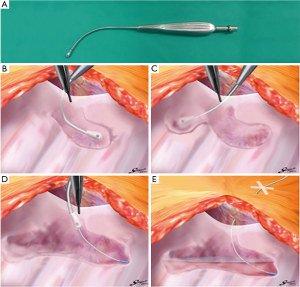
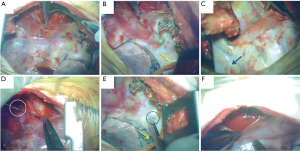
An epidural catheter is placed before the induction of general anesthesia and a PVB catheter is placed just before initiating the chest closure. We have described the procedure used to insert the PVB catheter during open chest surgery in detail in Figure S1. In this procedure, when the pleura is peeled off, a metal suction tube with a dull, curved tip is used (Figure 1A). At first, the parietal pleura is peeled back medially as far as the neck of the ribs at the thoracotomy opening. An extrapleural pocket is developed posteriorly and extends for 2–3 intercostal spaces above and below the level of the thoracotomy (Figure 1B,C). We usually peel the parietal pleura down to the 7th or 8th intercostal space so that the site of thoracoscopic port, which is also used for the drain insertion, should be anesthetized by this analgesia. An 18-gauge epidural catheter (typically used for epidural block: Epidural Catheterization Set, Allow International Inc., Pennsylvania, USA) is then placed percutaneously into this extrapleural space under direct visualization (Figure 1D). The final position of the catheter is with the lower portion in the part of the thoracic incision and the tip in the superior portion of the pocket (Figure 1E) (7,11,12). After the patient is transferred back to the recovery room, continuous infusion of 0.2% ropivacaine hydrochloride (Anapeine; Astra, Osaka, Japan) is started through an infusion pump (Figure 1E). Photographs of PVB catheter insertion in open chest surgery are shown in Figure 2.

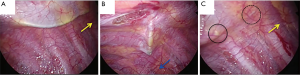
The procedure used to insert the PVB catheter in VATS has also been described in detail in Figure S1. As is the case in a standard thoracotomy, an indwelling extrapleural catheter is put in place just before the closure of each incision. At the posterior end of the thoracoscopic port, the parietal pleura is peeled off vertically with a metal suction tube over a range of 2–3 intercostal spaces above and below the level of the port (Figure 3A,B). Generally, we peeled off the parietal pleura so that all thoracoscopic ports could be anesthetized using a local anesthetic agent. The metal suction tube is slowly advanced into the pocket to bluntly dissect and lift away the parietal pleura from the inner chest wall to form an extrapleural pocket. An 18-gauge catheter is then placed percutaneously into this extrapleural space under thoracoscopic visualization (Figure 3C). In its final position, the lower portion of the catheter is within the inferior part of the pocket and the tip is in the superior portion of the pocket (4).
It is very important that the lifted parietal pleura is intact in the area of the extrapleural pocket so that the infused local anesthetic can easily spread throughout the extrapleural space without leaking through a torn area (Figures 1E,2F). Once the extrapleural pocket is filled, the local anesthetic induces an intercostal nerve blockade (Figures 1E,2F) (13). During catheter placement for PVB, pleural tear accidentally happened in 50 cases (13.6%, Table 1). In case of pleural disruption, we repaired it by patching Surgicel Nu-Knit® (ETHICON). In our study, as EPI, a bolus of 3.0 mL of 0.375% ropivacaine was administrated during surgery every 2 hours, followed by continuous administration of 3.0 mL/h of a compound of 0.1% ropivacaine and 0.004% fentanyl (Fentanyl Injection; Daiichi Sankyo, Tokyo, Japan) after surgery. As PVB, 5.0 mL bolus of 0.375% ropivacaine was administrated until the end of the operation (Figure 2F), followed by continuous administration of 3.0 mL/h of 0.2% ropivacaine after surgery. Every patient was encouraged to use extra painkiller by using patient-control-system of these analgesia. Ropivacaine was used for both EPI and PVB at set doses that would not cause systemic toxicity due to local anesthetic, even when the highest dose was administered using a patient-controlled system.
As an analgesic for the patients, a non-steroidal anti-inflammatory drug (NSAID) (Loxonin: loxoprofen sodium hydrate; Daiichi-Sankyo, Tokyo, Japan) was administered 3 times a day. In case of analgesic failure with NSAIDs, Pentagin (pentazocine hydrochloride; Daiichi-Sankyo, Tokyo, Japan) was administered intramuscularly as a rescue medication. We measured the analgesic efficacy of our method in terms of verbal rating scale and whether or not the rescue analgesic was given to supplement the efficacy of NSAIDs. Furthermore, the side effects related to the analgesia were also investigated. As a rule, both catheters were removed in the morning of 4th postoperative day.
Our method of PVB catheter insertion is a simple procedure that requires approximately 3 minutes and uses a metal suction with a blunt tip; we did not observe any patients experiencing severe complications, such as bleeding, with catheter insertion (Figure S1). No severe postoperative complications, including hypotension, bleeding, empyema, consciousness disorder, overmedication, or respiratory failure related to analgesia were observed. Thirty-two patients suffered from nausea, but the symptoms were improved by removing fentanyl from EPI, or finishing EPI. In fact, the EPI regimen was changed in 26 patients and discontinued in 6 patients.
We retrospectively assessed the number of postoperative uses of Pentagin (total) by reviewing the clinical records (Table 2). From October 2013 to October 2014, the mean [± standard deviation (SD)] number of uses was 2.8±1.2/patient after open chest surgery and 2.1±1.2/patient after VATS; the corresponding numbers from November 2014 to December 2016 were 0.3±0.1 and 0.2±0.1/patient, respectively (Table 2). The use of rescue medication was significantly reduced after introducing our combination method. However, the incidence of pneumonia and length of hospital stay after surgery were not significantly different between patients using our combination method and the historical control (Table 2).
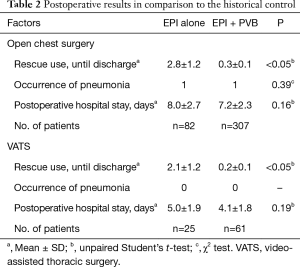
Full table
Pain was controlled to a level described as nonexistent-to-mild on the verbal rating scale in 306 patients (83.2%) for up to the fourth postoperative day. Interestingly, 241 patients (65.5%) reported the new onset of mild pain on or after the fourth postoperative day after catheter removal. The mean length of postoperative hospital stay was 7.2±2.3 days after open chest surgery and 4.1±1.8 days after VATS. All patients were ultimately discharged.
Comments
EPI has been most common analgesia method for thoracotomy pain due to its well-known effectiveness. However, EPI has a kind of adverse effects, which include urinary retention, nausea and hypotension. Also, it is reported that EPI’s failure rate is up to 12% (3), and has some limitations and contraindications such as anatomical difficulty and antiplatelet or anticoagulation usage. Recently, as an alternative analgesic method, PVB is reported to have good pain-control efficacy and fewer side effects. A number of studies that compare PVB with EPI for thoracotomy and meta-analysis studies reveal PVB provides comparable analgesia with EPI and has better side effect profile (6,8-10). Generally, EPI is blindly administered into the epidural space, and results in bilateral anesthesia. This type of therapy is likely to induce systemic side effects, such as nausea, urinary retention, and hypotension. Moreover, EPI can’t be administered to patients with bleeding tendencies. However, our PVB method allows catheter placement under direct visualization, provides only ipsilateral nerve block relative to the surgical site, and causes only a few systemic side effects. In addition, our method can be applied even to patients with bleeding tendencies. Although there are no doubts about the efficacies of these two methods, the patients after thoracotomy still feel moderate pain and require additional painkillers. In this context, we tried to manage post-operative pain after thoracotomy by the combination method of EPI and PVB.
In this study, there were no severe complications related to analgesia, however 32 patients could not tolerate conventional EPI because of nausea, leading to changes in the EPI regimens and discontinuation of EPI. In other words, the combination method allows flexible approaches such as changes in the EPI regimen and/or discontinuation of EPI because PVB alone provides sufficient analgesia and therefore acts as a safety net.
In 50 patients, pleural disruption was accidentally formed during PVB procedure. Komatsu et al. reported that the efficacy of the PVB depends somewhat on whether the procedure is done without pleural disruption or not (14). Although pleural disruption is expected to reduce the analgesic effects, EPI would be expected to exert complementary analgesic effects when administered via our combination method, even in such cases. In addition, the occurrence of pleural disruption was distributed equally over the observation period and was not frequently observed in the initial phase after initiating the PVB. Our PVB insertion technique is relatively simple and does not require a long training period to learn.
The rate of Pentagin use decreased after the introduction of our novel analgesic treatment. Moreover, patients' complaints of postoperative pain decreased dramatically. The finding that many patients reported new onset of pain on or after the fourth postoperative day after catheter removal indicates that pain had been largely controlled by double anesthesia during the acute postoperative stage. We interpret this to mean that our combination of EPI and PVB could yield more efficacious synergistic pain control than either EPI or PVB alone.
This study had several limitations. First, we did not use the visual analogue scale (VAS), which is the most commonly employed method for pain assessment (15). As a substitute for VAS, we used verbal rating scale and measured the degree of pain control according to the necessity of a rescue medication. Secondly, our analysis was retrospective and observational at a single institution.
This study is neither randomized nor comparative, but demonstrates safety and feasibility of the combination analgesia of EPI and PVB. At lease, from the view of clinical setting, satisfactory analgesia seems to be obtained by this novel method. Further investigation will be needed to validate our method and certify the superiority of this method over conventional single methods of EPI or PVB. In conclusion, the combination analgesia of EPI and PVB is safe and feasible, and seems effective.
Acknowledgements
The authors greatly appreciate Takuji Fujinaga and Teruya Komatsu for helpful scientific discussions.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethics board of Yamanashi Central Hospital. The patients provided written informed consent for the studies.
References
- Muehling BM, Halter GL, Schelzig H, et al. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. Eur J Cardiothorac Surg 2008;34:174-80. [Crossref] [PubMed]
- Pluijms WA, Steegers MA, Verhagen AF, et al. Chronic post-thoracotomy pain: a retrospective study. Acta Anaesthesiol Scand 2006;50:804-8. [Crossref] [PubMed]
- Hansdottir V, Philip J, Olsen MF, et al. Thoracic epidural versus intravenous patient-controlled analgesia after cardiac surgery: a randomized controlled trial on length of hospital stay and patient-perceived quality of recovery. Anesthesiology 2006;104:142-51. [Crossref] [PubMed]
- Komatsu T, Kino A, Inoue M, et al. Paravertebral block for video-assisted thoracoscopic surgery: analgesic effectiveness and role in fast-track surgery. Int J Surg 2014;12:936-9. [Crossref] [PubMed]
- Komatsu T, Sowa T, Takahashi K, et al. Paravertebral block as a promising analgesic modality for managing post-thoracotomy pain. Ann Thorac Cardiovasc Surg 2014;20:113-6. [Crossref] [PubMed]
- Richardson J, Sabanathan S, Jones J, et al. A prospective, randomized comparison of preoperative and continuous balanced epidural or paravertebral bupivacaine on post-thoracotomy pain, pulmonary function and stress responses. Br J Anaesth 1999;83:387-92. [Crossref] [PubMed]
- Sabanathan S, Smith PJ, Pradhan GN, et al. Continuous intercostal nerve block for pain relief after thoracotomy. Ann Thorac Surg 1988;46:425-6. [Crossref] [PubMed]
- Ding X, Jin S, Niu X, et al. A comparison of the analgesia efficacy and side effects of paravertebral compared with epidural blockade for thoracotomy: an updated meta-analysis. PLoS One 2014;9:e96233. [Crossref] [PubMed]
- Scarfe AJ, Schuhmann-Hingel S, Duncan JK, et al. Continuous paravertebral block for post-cardiothoracic surgery analgesia: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2016;50:1010-8. [Crossref] [PubMed]
- Yeung JH, Gates S, Naidu BV, et al. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev 2016;2:CD009121. [PubMed]
- Sabanathan S, Richardson J, Shah R. 1988: Continuous intercostal nerve block for pain relief after thoracotomy. Updated in 1995. Ann Thorac Surg 1995;59:1261-3. [Crossref] [PubMed]
- Watson DS, Panian S, Kendall V, et al. Pain control after thoracotomy: bupivacaine versus lidocaine in continuous extrapleural intercostal nerve blockade. Ann Thorac Surg 1999;67:825-8; discussion 828-9. [Crossref] [PubMed]
- Detterbeck FC. Efficacy of methods of intercostal nerve blockade for pain relief after thoracotomy. Ann Thorac Surg 2005;80:1550-9. [Crossref] [PubMed]
- Komatsu T, Sowa T, Kino A, et al. The importance of pleural integrity for effective and safe thoracic paravertebral block: a retrospective comparative study on postoperative pain control by paravertebral block. Interact Cardiovasc Thorac Surg 2015;20:296-9. [Crossref] [PubMed]
- Andreetti C, Menna C, Ibrahim M, et al. Postoperative pain control: videothoracoscopic versus conservative mini-thoracotomic approach. Eur J Cardiothorac Surg 2014;46:907-12. [Crossref] [PubMed]
- Yokoyama Y, Nakagomi T, Shikata D, et al. The procedure used to insert the PVB catheter during open chest surgery and VATS. Asvide 2017;4:257. Available online: http://www.asvide.com/articles/1566


