Incidence and risk factors for acute lung injury after open thoracotomy for thoracic diseases
Introduction
Owing to the improvements in patient selection, operative and anesthesia skills, and intensive care management, morbidities and mortality associated with thoracotomy have decreased dramatically in recent decades (1-3). However, acute lung injury (ALI), defined as hypoxemia accompanied by radiographic pulmonary infiltration without a clearly identifiable cause (4), remains a major complication after thoracic surgery (2,5,6). And it has become the leading cause of death postoperatively. While ALI is more frequently seen in elderly patients and those with diminished pulmonary function, recently there is increasing consensus that it originates at least partly from the traumatic responses imposed by major surgical procedures (7,8). Holding the belief that a better understanding of perioperative factors for ALI may help identifying high risk patients and thus lead to better preventive and management strategies, we undertook this prospective study to examine the incidences and possible risk factors for ALI in thoracotomy candidates.
Patients and methods
Among 364 consecutive open thoracotomies in an 18 months period, there were 127 elderly patients (35%) over 65 years of age. Fifty-eight of them were considered at potential risk of developing ALI, either because they were super-geriatric (aged over 75 years), with compromised cardiopulmonary function, obese (BMI >28), or having primary lesions necessitating extremely devastating procedures such as pneumonectomy or esophagectomy. These 58 elderly patients were prospectively entered into the study group. Fifty-six patients under 65 years of age were prospectively selected as the control group, matched to the study group by surgical procedures. Since the study was observational in nature and perioperative management was designed so as not to be affected by the results during the study, it was approved by the Ethics Committee of the hospital and confirmed consent from individual patient was waived.
Preoperative workup included both functional evaluation and clinical staging if malignancy was suspected. The criteria of the American Thoracic Society (9) and spirometry results (FEV1/FVC ≤70% of predicted value) were used for the diagnosis of chronic obstructive pulmonary disease (COPD). Standard anesthesia methods and postoperative care were used in all patients. The intention of surgery was for curative resection of the lesion in all cases, with systemic lymph node dissection when the underlying disease was proved malignant.
Perioperative respiratory and circulatory status was closely monitored and recorded. These included routine echocardiographic changes, arterial blood pressure, central venous pressure, pulse oximetry (SpO2), as well as detailed fluid intake and output. Arterial blood gas on room air was checked daily before and three days after operation. All adverse events during or after operation were documented in detail for further analysis.
Primary ALI was defined as acute onset of hypoxemia (with a Pao2/Fio2 ratio of <300) with subsequent radiographic infiltrates characteristic of pulmonary edema, according to American-European Consensus Conference on ARDS guidelines (4). These included acute pneumonitis, respiratory distress, as well as ARDS. Respiratory insufficiency secondary to any surgical complications such as massive bleeding, anastomotic breaking down, or with predisposing conditions like empyema or systemic infection, were excluded.
Results from the two groups were compared using SSPS 13.0 software (SPSS Inc, Chicago, IL), with P values less than 0.05 considered of statistical significance. Categorical variants were examined with χ2 test or Fisher’s exact test when appropriate. Continuous variants were compared using Independent-samples t test. Multivariate risk factor analysis was carried out using Binary logistic regression model.
Results
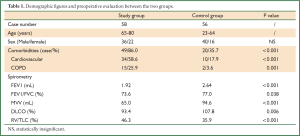
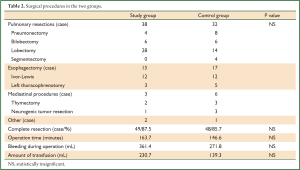
Apart from the age difference, patients in the study group had significantly more cardiopulmonary comorbidities and poorer spirometry data than the control group (Table 1). No difference was detected in complete resection rates, time of operation, or amount of bleeding and infusion between the two groups (Table 2).
Full Table
Full Table
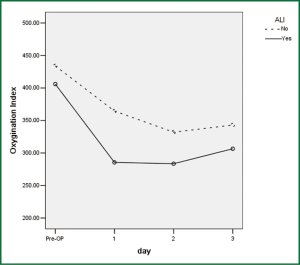
Significantly more postoperative complications occurred in the elderly group (58.6%) than in the control group (17.9%, P<0.001), especially morbidities related to major organ dysfunction (51.7% vs. 12.5%, P<0.001). ALI occurred in 10 patients, exclusively in the elderly group, on the second to third postoperative days (Figure 1). None of the patients in the control group or in those not enrolled into the study experienced ALI. The occurrence rate of ALI was 2.7% in the whole series (10/364), and 7.9% in elderly patients (10/127). Six of the 10 ALI patients underwent lobectomy and the other 4 esophagectomy. It led to 3 deaths due to respiratory failure in spite of vigorous resuscitation. There was one additional death from aspiration two weeks after thoracotomy in the study group. So the mortality for patients with ALI was 30%, significantly higher than those without ALI (1.0%, P=0.001). Technical morbidities directly related to surgical maneuver occurred similarly in the two groups (13.8% vs. 7.1%, P=0.362). There were no significant difference in overall mortality rates between the two groups (6.9% vs. 0%, P=0.119).
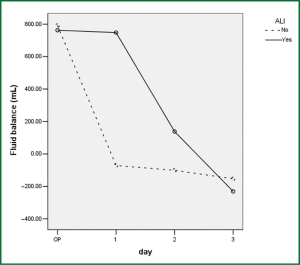
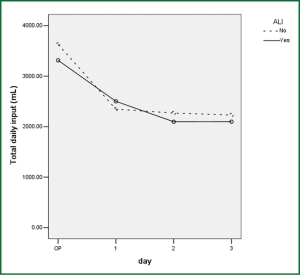
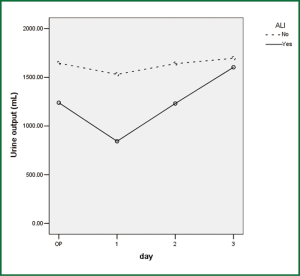
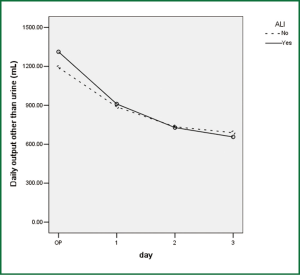
Comparison of perioperative fluid balance showed that patients with ALI experienced significantly positive water balance on the first operative day, when in patients without ALI it was slightly negative (Figure 2). On the operative day, however, there was no difference between the two groups. Amount of fluid administration was comparable on the first postoperative day (Figure 3), and so was the amount of output other than urine (Figure 4). Consequently, it was the significant difference in urine output (Figure 5) that constituted the major difference in total output, and therefore fluid balance, on the first postoperative day between patients with or without ALI. Unlike on postoperative day 1, there was no significant difference in fluid balance between patients with or without ALI on the day of operation or on postoperative day 2 and 3.
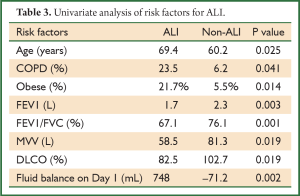
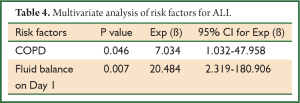
Upon univariate analysis, increased age, obesity, COPD, poor spirometry (including FEV1, FEV1/FVC, MVV, RV/TLC, and DLCO), and positive fluid balance on postoperative day 1 were associated with increased risk of developing ALI after thoracotomy (Table 3). Upon multivariate analysis, however, only COPD and excessive positive fluid balance on postoperative day 1 were revealed as independent risk factors for ALI (Table 4).
Full Table
Full Table
Comment
This study suggests that ALI is still a major morbidity and the leading cause of death after open thoracotomy, especially in elderly patients. While the incidence of ALI was repeatedly reported to be 2-8% after lung resection (7,8,10), few series has included other surgery such as esophagectomy and mediastinal procedures. In the current study it was 2.7%, equally distributed between patients undergone lung and esophageal resections. Lesser surgery like segmentectomies was also included in this study as similar incidence of ALI has been reported (8), probably because the decision on sublobar resection might be related to poor functional status. The outcome of patients with ALI was unanimously dismal in literature, with significant mortality rates ranging from 40-100% (7,8,10,11). In the current series it was 30%, a little lower than reported but still significantly higher than in patients without ALI.
Apart from careful patient selection, meticulous surgical maneuver, and vigilant postoperative care, the somewhat lower incidence and mortality of ALI in our patients comparing with the existing literature reflects the difference in the definition of postoperative ALI. Although the number of reports on this devastating syndrome keeps increasing, identification of ALI has been obscured by the lack of proper diagnostic criteria and by the retrospective nature in most studies. Licker et al. (10) recently reported 37 ALI after lung resection, 4.2% in 879 patients with a mortality rate of 35.1%. Further careful study revealed that 10 of them were late onset (3-12 days after operation), secondary to complications like bronchogenic pneumonia, bronchopleural fistula, or gastric aspiration. A mortality of 60% was associated with this kind of secondary ALI. After excluding these 10 patients, the incidence and mortality of primary ALI (with no confounding cause and occurred exclusively during 0-3 postoperative days) was reduced to 3.1% and 26%, respectively. The authors once also studied the incidence of respiratory complications, though not strictly defined ALI, after esophagectomy with 3-field lymph node dissection and got similar results (5). Rates of primary and secondary pulmonary complications were 4.3% and 3.9%, respectively. And the associated mortality of primary pulmonary complications was 15.8%, significantly lower than that of secondary ones, being 47.1%. In the current series, all ALI occurred within 3 days after thoracotomy with no disposing conditions, in accordance with Licker’s observation. Because this study was prospectively designed, complete data of blood gas from all patients were available to avoid any underestimation of the true incidence of ALI. And all blood gas was checked under room air condition to avoid the possible influence of inhaled oxygen concentration. Therefore, we believe the results reflect the true incidence and mortality of ALI after thoracotomy in a contemporary setting. Still ALI turned out to be the main cause of mortality in our patients.
It has been well documented that diminished pulmonary functional status is associated with increased morbidity after thoracotomy (10,12). Recently, Alam et al. (13) further revealed from retrospective analysis that decreased postoperative predicted lung function, especially diffusion capacity, was an independent risk factor for ALI after lung resection. The odds ratio of this risk was quantified to be 1.10 for each decrease of 5% in DLco. In the current study, decrease in almost all spirometry indexes was related to increased risk of postoperative ALI. However, only preoperative COPD was identified as an independent risk factor. Although all ALI occurred in the elderly group and increased age was associated with ALI on univariate analysis, age itself did not turn out to be an independent risk factor on multivariate analysis. Since significantly more elderly patients had COPD than those in the control group, increased age was more likely a confounding factor. Evaluation of predicted postoperative lung function was not routinely required in this study. But ventilation/perfusion scan has long been in our armory of preoperative workup, especially for high risk thoracotomy candidates. Our results and those from Alam et al. (13) only further strengthened the importance of careful lung function risk assessment before going on to open thoracotomy.
Apart from patient selection, few studies have been focused on any possible relationship between perioperative management and ALI after thoracotomy. Zeldin et al. (14) first proposed that excessive perioperative fluid administration might promote lung injury after pneumonectomy. In Licker’s study, excessive fluid administration, high intraoperative ventilatory pressures were both independent risk factors for ALI (10). Alam et al. also found increasing fluid administration along with decreasing predicted postoperative lung function to be significant predictive factors for ALI after lung resection (13). The odds ratio was further quantified to be 1.17 for each 500 mL increment in perioperative fluid administered. The results from the current study confirm with the above reports in that excessively positive fluid balance carries a significantly higher risk of developing ALI after thoracotomy. However, it is also noteworthy that a major difference exists between those trials and the current one in the timing of study. While the above two reports focused on fluid administration on the day of operation [12-hour period right after operation in Alam’s trial (13) and including fluid given during anesthesia in Licker’s trial (10)], the current study covered a much wider time window from the operative day to 3 days after. Significant difference in fluid balance between patients with or without ALI was detected on the first postoperative day, instead of during or right after anesthesia on the day of operation. What is more, not only the fluid administered but also the detailed daily output was studied in the current series. Because of the observational nature of our study, the amount of fluid administered in patients having ALI was in fact equivalent to those without ALI. The significant difference of fluid balance on the first postoperative day lay in the amount of output, and in the urine output exclusively.
So the previous studies clearly carry a question concerning the mechanism of ALI, i.e., whether increased fluid load causes ALI. The question could be translated into whether decreased urine output plays a role in the development of ALI, according to the results from the current study. It should help determine whether any intervention can decrease the incidence of ALI, or help patients to better cope with the condition once it happens. Even fewer studies have been performed based on this concern. A recently published ARDS trial (15) compared the effect of a conservative or a liberal strategy of fluid management in patients with ALI. Although there was no significant difference in mortality, the conservative strategy improved lung function and shortened the duration of mechanical ventilation and intensive care. In the current study, a similarly liberal strategy of fluid administration was followed, based on the well-established concept of pathogenic response to traumatic injury imposed by open thoracotomy (16,17). Such liberal strategy would be rational to achieve hemodynamic optimization at the early stage of surgical trauma characterized by low cardiac output and poor tissue perfusion. Upon entering the second stage, so called ‘the flow phase’, the swollen patient has an increased cardiac output, a mobilization of extravascular fluid, normal tissue perfusion where diuresis usually occurs. From the results of the current study, it is reasonable to relate ALI and implied pulmonary edema to insufficient volume depletion, manifested by less urine output in the early postoperative days. Therefore, conservative fluid strategies, or the application of diuretics, are therapeutically sound (18). As the transition from the first stage to ‘the flow phase’ may be indistinct, the timing of the initiation of conservative fluid management is very important. Based on the results of the current study, it would be most appropriate to start on the first postoperative day, instead of during or immediately after anesthesia. And caution should always be borne to preserve organ perfusion and avoid metabolic derangement (15,18).
In conclusion, ALI remains a significant source of morbidity and mortality after open thoracotomy. COPD and excessive positive fluid balance on the first postoperative day are independent risk factors associated with ALI. Preventative and interventional strategies like rightly timed conservative fluid administration or diuretic therapy may help minimize this potentially devastating complication after major thoracic surgery.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Damhuis RA, Schütte PR. Resection rates and postoperative mortality in 7,899 patients with lung cancer. Eur Respir J 1996;9:7-10. [PubMed]
- Watanabe S, Asamura H, Suzuki K, et al. Recent results of postoperative mortality for surgical resections in lung cancer. Ann Thorac Surg 2004;78:999-1002; discussion 1002-3. [PubMed]
- Low DE, Kunz S, Schembre D, et al. Esophagectomy--it’s not just about mortality anymore: standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. J Gastrointest Surg 2007;11:1395-402; discussion 1402. [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [PubMed]
- Slinger PD. Acute lung injury after pulmonary resection: more pieces of the puzzle. Anesth Analg 2003;97:1555-7. [PubMed]
- Fang W, Kato H, Tachimori Y, et al. Analysis of pulmonary complications after three-field lymph node dissection for esophageal cancer. Ann Thorac Surg 2003;76:903-8. [PubMed]
- Kutlu CA, Williams EA, Evans TW, et al. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg 2000;69:376-80. [PubMed]
- Ruffini E, Parola A, Papalia E, et al. Frequency and mortality of acute lung injury and acute respiratory distress syndrome after pulmonary resection for bronchogenic carcinoma. Eur J Cardiothorac Surg 2001;20:30-6, discussion 36-7. [PubMed]
- Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med 1995;152:S77-121. [PubMed]
- Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 2003;97:1558-65. [PubMed]
- Dulu A, Pastores SM, Park B, et al. Prevalence and mortality of acute lung injury and ARDS after lung resection. Chest 2006;130:73-8. [PubMed]
- Parquin F, Marchal M, Mehiri S, et al. Post-pneumonectomy pulmonary edema: analysis and risk factors. Eur J Cardiothorac Surg 1996;10:929-32; discussion 933. [PubMed]
- Alam N, Park BJ, Wilton A, et al. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 2007;84:1085-91; discussion 1091. [PubMed]
- Zeldin RA, Normandin D, Landtwing D, et al. Postpneumonectomy pulmonary edema. J Thorac Cardiovasc Surg 1984;87:359-65. [PubMed]
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564-75.
- Cuthbertson DP, Angeles Valero Zanuy MA, León Sanz ML. Post-shock metabolic response. 1942. Nutr Hosp 2001;16:176-82; discussion 175-6. [PubMed]
- Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS). Ann Intern Med 1996;125:680-7. [PubMed]
- Rivers EP. Fluid-management strategies in acute lung injury--liberal, conservative, or both? N Engl J Med 2006;354:2598-600. [PubMed]