Recurrent laryngeal nerve injury after esophagectomy for esophageal cancer: incidence, management, and impact on short- and long-term outcomes
Introduction
Esophageal cancer is the eighth most common type of cancer worldwide, and its incidence is rising (1-3). The current standard of care for patients with a resectable locally advanced tumor is neoadjuvant chemo(radio)therapy, followed by surgical resection with a radical lymphadenectomy (4-6).
During esophagectomy, thermal injury, stretching, compression, or vascular compromise of the recurrent laryngeal nerve (RLN) may cause RLN palsy (RLNP) (7). The incidence of RLNP after esophagectomy varies, ranging from 0% to 59% (8-18). These disparities have been attributed to variation in extent of lymph node dissection, surgical technique (two- or three stage), the size and T-stage of the primary tumor, and the means of RLN injury diagnosis (8,19-21).
In addition to being the most important motor nerve of the larynx, the RLN innervates the cricopharyngeal muscle which form the upper esophageal sphincter, hereby playing a central role in swallowing (22). Patients with RLNP may present with symptoms ranging from hoarseness, dyspnea during speech, aspiration, difficulty with coughing, marked morbidity through pulmonary complications [pneumonia, acute respiratory distress syndrome (ARDS), atelectasis], and may even suffocate in case of bilateral damage. Only few retrospective studies have reported on the consequences of RLNP on the short term, particularly the incidence of pulmonary complications (8-10,19,23,24). Furthermore, information on the long term (i.e., recovery and possible surgical interventions) is lacking. Therefore, the current study aims to evaluate the consequences of RLNP in terms of (pulmonary) morbidity and long-term functional recovery.
Methods
Patients
This cohort study used a prospective database of the University Medical Center Utrecht (UMCU) to include patients who underwent a 3-stage transthoracic (McKeown) or transhiatal esophagectomy with a gastric conduit reconstruction for esophageal carcinoma between January 2004 and March 2016. Specific follow-up data regarding RLNP were supplemented from the electronic patient record. This study received ethical approval (Institutional Review Board number 13-061/C) from the Medical Ethics Review Committee of the UMCU, and informed consent was waived.
Outcomes
Primary outcome was the association of RLNP and postoperative pulmonary complications. To create a homogeneous cohort for analyzing the association between RLNP and postoperative pulmonary complications, only the McKeown esophagectomies were included. RLNP was defined as any kind of damage inflicted during surgery to the left, the right, or both of the RLN (s), resulting in paresis or paralysis. Paresis was defined as a partial interruption of laryngeal innervation, leading to hypomobility of the laryngeal muscles. Paralysis was defined as no motion of the affected muscle(s) (25). Pulmonary complications were defined as clinically proven pneumonia [in accordance with the revised Uniform Pneumonia Score (26)], pleural effusion leading to drainage, pleural empyema, ARDS, atelectasis, re-intubation, or the need for a tracheostomy.
Secondary outcomes were otolaryngological consultation for RLNP, clinical presentation of the RLNP (dysphonia and/or aspiration), means of RLNP diagnosis (clinical or laryngoscopic), and RLNP-specific therapy and functional recovery. For functional recovery, only people with a follow-up of at least 6 months at the time of data extraction were included, because RLNP recovery may not have occurred before that time (27). Complete recovery from RLNP symptoms, possibly with laryngoscopy showing full revival of vocal cord mobility, was seen as full recovery. Clinical improvement of RLNP symptoms, possibly with laryngoscopy showing some improvement of vocal cord mobility, was seen as partial recovery. No improvement of RLNP symptoms, possibly with laryngoscopy showing no improvement of vocal cord mobility, was seen as no recovery. Other secondary outcomes included re-intervention, 30-day postoperative or in-hospital mortality, intensive care unit (ICU) and hospital stay in days, readmission within 30 days, non-radical resection, and anastomotic leakage.
Statistical methods
All data were analyzed using IBM SPSS Statistics for Windows, version 22.0 (IBM corp., Armonk, New York, USA). All continuous data were presented as median (range) or mean [± standard deviation (SD)] based on their distribution; all categorical data were presented as a number (percentage). Baseline data were analyzed using either chi-square tests (categorical data), Mann-Whitney-U tests, or student’s t-test (continuous data). Multivariable logistic and linear analyses were conducted to assess the association between RLNP and pulmonary complications and hospital stay, respectively. All variables with a P value <0.2 in univariable analysis were entered in the multivariable analyses and a P value of <0.05 was considered statistically significant.
Results
Patients
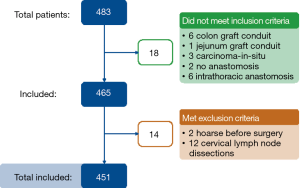
Between January 2004 and March 2016, 324 underwent a 3-stage transthoracic (McKeown) esophagectomy with a two-field lymphadenectomy and 127 patients underwent a transhiatal esophagectomy for esophageal carcinoma (Figure 1).
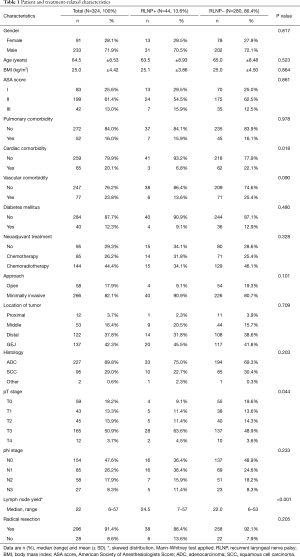
The baseline data showing patient and treatment-related characteristics for the McKeown group are provided in Table 1. The mean (SD) age was 65 (±8.53) years, 72% of the patients were male, most (61%) had an ASA II status and 51% were diagnosed with a pT3 tumor. The majority (82%) of patients underwent robot-assisted thoraco laparoscopic minimally invasive surgery (da Vinci Si System, Intuitive Surgical Inc., Sunnyvale, CA, USA), the other 58 (18%) underwent open surgery.
Full table
RLNP
In the McKeown group, 44 of the 324 patients (14%) were found to have a postoperative RLNP (the RLNP+ group). Interestingly, in the cohort of transhiatal esophagectomies RLNP occurred in only 3/127 (2%) patients.
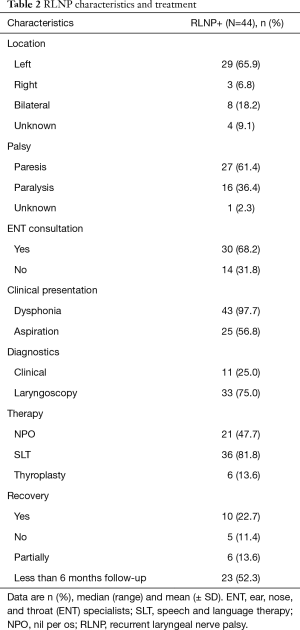
In our study population of McKeown esophagectomies: 33/44 RLNPs were diagnosed through laryngoscopy, 11/44 RLNP patients were diagnosed by clinical examination (Table 2). Most patients (29/44) presented with a left RLNP, 3/44 had a right RLNP, 8/44 had bilateral palsy, and for 4/44 patients the location was unknown. The majority of patients were diagnosed with a paresis (27/44); the most common symptoms at presentation were dysphonia (43/44) and/or aspiration (25/44). Of all patients diagnosed with RLNP, the majority (36/44) received speech and language therapy (SLT), 21/44 were kept nil-per-os (NPO).
Full table
Lymph node yield was higher in the RLNP+ group {24.5 [7–57] versus 22 [6–53] in the RLNP− group, P<0.001}. There was also a significant difference in the pathological T-stage between the two groups (P=0.044), with more T3–T4 tumors in the RLNP+ group and more T0–T2 tumors in the RLNP− group, as is shown in Table 1.
Postoperative outcomes
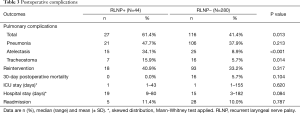
In the univariable analysis, there were significantly more postoperative pulmonary complications in the RLNP+ group (61% versus 41% in the RLNP− group; P=0.013) (Table 3). Specifically, there was a significantly higher amount of atelectasis (34% versus 9%; P<0.001) and tracheostomies performed (16% versus 6%; P=0.014) in the RLNP+ group. The difference in length of hospital stay between the RLNP+ and RLNP− groups was not statistically significant (19 versus 15 days, respectively; P=0.084).
Full table
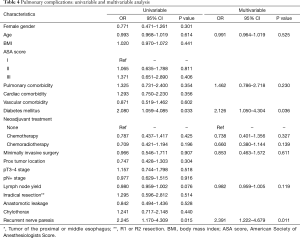
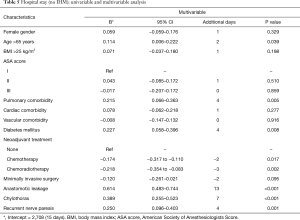
Results from the multivariable analyses are shown in Tables 4 and 5. RLNP was associated with a higher incidence of pulmonary complications (Table 4) (OR 2.391; 95% CI 1.222–4.679; P=0.011). In addition, diabetic comorbidity was also associated with a higher incidence of pulmonary complications (OR 2.126; 95% CI 1.050–4.304; P=0.036). Moreover, RLNP was associated with a prolonged hospital stay (+4 days) (P=0.001, Table 5). Other factors associated with a longer hospital stay were older age (above 65 years) (P=0.039), pulmonary comorbidity (P=0.005), diabetes mellitus (P=0.008), anastomotic leakage (P<0.001), chylothorax (P<0.001). Factors associated with a shorter hospital stay were the ability to receive neoadjuvant chemotherapy (P=0.017) or chemoradiotherapy (P=0.002).
Full table
Full table
Functional outcomes
The RLNP+ group is further characterized in Table 2. For functional outcome analysis only patients with a follow-up of at least 6 months were included (n=21). Median follow-up was 17.5 [7–135] months. During follow-up, almost half of patients made a full recovery (10/21), 5/21 patients recovered partially, and 6/21 patients did not recover. For 6/21 of these patients surgical intervention (medialization thyreoplasty) was required. All treatment interventions for vocal cord paralysis were conducted after a median time of 16.5 [11–29] months after esophagectomy. Of all patients that underwent medialization thyreoplasty, 3/6 had no more RLNP symptoms afterwards 2/6 made a partial recovery from their RLNP, and 1/6 did not recover.
Discussion
The present study demonstrates that RLNP after esophagectomy was an independent predictor for both pulmonary complications and increased hospital stay. Moreover, it shows that over half of patients with RLNP after esophagectomy did not fully recover during follow-up, and a substantial part (14%) needed a surgical intervention to recover from RLNP.
These results stress the importance of preserving the RLN for both short- and long-term outcomes, since several studies have demonstrated that pulmonary complications significantly increase the ICU readmission rate, length of hospital stay and mortality rate, and permanent RLNP after esophagectomy deteriorates quality of life (15,28,29).
Similar to other Western studies reporting on RLNP after McKeown esophagectomy with a two-field lymph node dissection and cervical anastomosis, this study found a RLNP incidence of 14%. Nevertheless, most RLNPs were temporary, indicating that injury of the RLN is rather caused by indirect actions like compression or traction of the nerve than direct damage. Asian studies report RLNP incidences up to 59% (8,9,13,16). This is mainly attributed to the extensive three-field lymph node dissection which is standard of care in Asia. Notably, our data also showed that patients with RLNP had a significantly higher lymph node yield, possibly indicating a more extensive lymph node dissection in these patients. Also, in this series, in the three stage procedures, a level 2 and 4 (paratracheal) lymph node dissection was always performed.
The results of this study regarding short-term complications after RLN injury during esophagectomy are in line with current literature (9,10,20,23,24). The first study published on this topic found higher rates of pulmonary complications after RLNP, leading to a higher reintubation rate, and consequently a prolonged ventilation time and longer ICU stay (23).
These findings were confirmed in more recent studies, all demonstrating an increase in pulmonary complications after esophagectomy complicated by RLNP (9,10,20,24). Similarly to our results, Koyanagi et al. (10) found an association between RLNP and prolonged hospital stay. No other studies could confirm this finding, though these studies were limited by their small sample sizes.
This study demonstrates that preventing RLNP during esophagectomy is not only pivotal for improving short-term, but also long-term surgical outcomes. The only study in which results regarding RLNP recovery after esophagectomy are published to date focused on quality of life 1 year after RLNP and found a significant deteriorated quality of life after permanent nerve paralysis due to esophagectomy (12). Hence, precautions to prevent RLNP can improve the outcomes after esophagectomy.
Considering its harmful consequences, it is important to find ways to prevent RLNP during esophagectomy. After transhiatal esophagectomy, the RLNP incidence was 2%, while this was 14% after McKeown esophagectomy. This indicates that the RLN is at risk during high mediastinal lymph node dissection. In accordance with current literature, the majority of patients in the present study were diagnosed with a left-sided palsy (10,20). The left RLN is longer than the right RLN and is situated close to lymph node stations 2L and 4L, consequently being more at risk for injury during lymph node dissection. Meticulous dissection of these stations is pivotal since there is a high frequency of lymph node metastasis (30-32). We experience robotic assistance of great value to perform a full paratracheal lymph node clearance, which was the standard of care for all these patients (33,34). Robotic assistance facilitates meticulous dissection along the left RLN and may reduce the incidence of RLNP (14,35).
Surgically induced RLNP is often not recognized during the procedure. Noninvasive intraoperative neurological monitoring (IONM) may enable surgeons to identify and preserve the RLN. IONM is already widely used in thyroid surgery, and although its effectiveness for esophagectomy is not well recognized, several studies report lower RLNP and consequently lower pulmonary complication rates (8,20,36-38). Therefore, the use of IONM may be considered for esophagectomy, particularly during high mediastinal lymph node dissection (10). A substantial part of RLNP is due to thermal injury. Electrocautery devices are used for hemostasis around the RLN during esophagectomy and may deliver heat at a single temperature or a range of temperatures, between 100 and 1,200 °C. The use of alternatives to an electrocautery device, reducing maximum temperatures may be another measure to reduce RLNP rates (39). Additionally, the use of an intrathoracic anastomosis instead of a cervical anastomosis may also reduce the incidence of RLNP after esophagectomy (20,21), most probably because of the fact that the upper mediastinum is not fully dissected in these cases.
Besides avoiding unnecessary RLNP, early diagnosis might prevent complications secondary to RLNP. Over half of patients in the current study were diagnosed with RLNP after clinical presentation with symptoms of aspiration. In case of early diagnosis, one may decide to properly evaluate the swallowing process and potentially postpone oral intake, reducing aspiration pneumonia rates (20).
The majority of patients underwent SLT after hospital discharge, resulting in a satisfactory function of voice and swallowing. However, due to persistent symptoms, 14% of all RLNP patients needed secondary surgery (medialization thyroplasty), leading to a full or partial recovery in 5 out of 6 patients. All procedures were conducted in the absence of spontaneous recovery ≥10 months after esophagectomy. This suggests that these patients may have benefited from earlier intervention, since several studies on early versus late medialization show favorable outcomes for patients in early cohorts (40,41).
Strengths of this study include its large sample size from a prospective database of Western patients and the detailed information on both short- and long-term outcomes available from the database. Despite this, certain limitations apply to the current analysis. It was not possible to obtain data on recovery in all patients. All patients in which follow-up data was missing had early recurrent disease. This made complaints regarding RLNP of minor importance, resulting in poor registration. Lastly, an unexpected lower rate of cardiac comorbidity was found in the RLNP+ group. Although multivariable analysis corrected for all known confounders such as cardiac comorbidity, there may have been other unexpected, unknown confounders which were not included in the analysis, which may have led to bias.
The present study shows that RLNP after esophagectomy is associated with an increased pulmonary complication rate, longer hospital stay, and a moderate long-term recovery. This warrants further studies examining technologies that may reduce RLNP rates.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study received ethical approval (Institutional Review Board number 13-061/C) from the Medical Ethics Review Committee of the UMCU, and informed consent was waived.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598-606. [Crossref] [PubMed]
- Dikken JL, Lemmens VE, Wouters MW, et al. Increased incidence and survival for oesophageal cancer but not for gastric cardia cancer in the Netherlands. Eur J Cancer 2012;48:1624-32. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Myssiorek D. Recurrent laryngeal nerve paralysis: anatomy and etiology. Otolaryngol Clin North Am 2004;37:25-44. v. [Crossref] [PubMed]
- Gelpke H, Grieder F, Decurtins M, et al. Recurrent laryngeal nerve monitoring during esophagectomy and mediastinal lymph node dissection. World J Surg 2010;34:2379-82. [Crossref] [PubMed]
- Gockel I, Kneist W, Keilmann A, et al. Recurrent laryngeal nerve paralysis (RLNP) following esophagectomy for carcinoma. Eur J Surg Oncol 2005;31:277-81. [Crossref] [PubMed]
- Koyanagi K, Igaki H, Iwabu J, et al. Recurrent Laryngeal Nerve Paralysis after Esophagectomy: Respiratory Complications and Role of Nerve Reconstruction. Tohoku J Exp Med 2015;237:1-8. [Crossref] [PubMed]
- Nishimaki T, Suzuki T, Suzuki S, et al. Outcomes of extended radical esophagectomy for thoracic esophageal cancer. J Am Coll Surg 1998;186:306-12. [Crossref] [PubMed]
- Baba M, Natsugoe S, Shimada M, et al. Does hoarseness of voice from recurrent nerve paralysis after esophagectomy for carcinoma influence patient quality of life? J Am Coll Surg 1999;188:231-6. [Crossref] [PubMed]
- Pertl L, Zacherl J, Mancusi G, et al. High risk of unilateral recurrent laryngeal nerve paralysis after esophagectomy using cervical anastomosis. Eur Arch Otorhinolaryngol 2011;268:1605-10. [Crossref] [PubMed]
- Suda K, Ishida Y, Kawamura Y, et al. Robot-assisted thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve for esophageal squamous cell carcinoma in the prone position: technical report and short-term outcomes. World J Surg 2012;36:1608-16. [Crossref] [PubMed]
- Baba M, Aikou T, Yoshinaka H, et al. Long-term results of subtotal esophagectomy with three-field lymphadenectomy for carcinoma of the thoracic esophagus. Ann Surg 1994;219:310-6. [Crossref] [PubMed]
- Walther B, Johansson J, Johnsson F, et al. Cervical or Thoracic Anastomosis After Esophageal Resection and Gastric Tube Reconstruction A Prospective Randomized Trial Comparing Sutured Neck Anastomosis With Stapled Intrathoracic Anastomosis. Ann Surg 2003;238:803-12; discussion 812-4. [Crossref] [PubMed]
- Fujita H, Kakegawa T, Yamana H, et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg 1995;222:654-62. [Crossref] [PubMed]
- Wright CD, Zeitels SM. Recurrent laryngeal nerve injuries after esophagectomy. Thorac Surg Clin 2006;16:23-33. v. [Crossref] [PubMed]
- Sato Y, Kosugi S, Aizawa N, et al. Risk Factors and Clinical Outcomes of Recurrent Laryngeal Nerve Paralysis After Esophagectomy for Thoracic Esophageal Carcinoma. World J Surg 2016;40:129-36. [Crossref] [PubMed]
- Taniyama Y, Miyata G, Kamei T, et al. Complications following recurrent laryngeal nerve lymph node dissection in oesophageal cancer surgery. Interact Cardiovasc Thorac Surg 2015;20:41-6. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Hammond CS, Davenport PW, Hutchison A, et al. Motor innervation of the cricopharyngeus muscle by the recurrent laryngeal nerve. J Appl Physiol (1985) 1997;83:89-94. [PubMed]
- Hulscher JB, van Sandick JW, Devriese PP, et al. Vocal cord paralysis after subtotal oesophagectomy. Br J Surg 1999;86:1583-7. [Crossref] [PubMed]
- Loochtan MJ, Balcarcel D, Carroll E, et al. Vocal Fold Paralysis after Esophagectomy for Carcinoma. Otolaryngol Head Neck Surg 2016;155:122-6. [Crossref] [PubMed]
- Rubin AD, Sataloff RT. Vocal fold paresis and paralysis. Otolaryngol Clin North Am 2007;40:1109-31. viii-ix. [Crossref] [PubMed]
- Weijs TJ, Seesing MF, van Rossum PS, et al. Internal and External Validation of a multivariable Model to Define Hospital-Acquired Pneumonia After Esophagectomy. J Gastrointest Surg 2016;20:680-7. [Crossref] [PubMed]
- Woodson GE, Miller RH. The timing of surgical intervention in vocal cord paralysis. Otolaryngol Head Neck Surg 1981;89:264-7. [Crossref] [PubMed]
- van der Sluis PC, Verhage RJ, van der Horst S, et al. A new clinical scoring system to define pneumonia following esophagectomy for cancer. Dig Surg 2014;31:108-16. [Crossref] [PubMed]
- Markar S, Gronnier C, Duhamel A, et al. Pattern of Postoperative Mortality After Esophageal Cancer Resection According to Center Volume: Results from a Large European Multicenter Study. Ann Surg Oncol 2015;22:2615-23. [Crossref] [PubMed]
- Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994;220:364-72; discussion 372-3. [Crossref] [PubMed]
- Tachimori Y, Nagai Y, Kanamori N, et al. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis Esophagus 2011;24:33-8. [Crossref] [PubMed]
- Matsubara T, Ueda M, Abe T, et al. Unique distribution patterns of metastatic lymph nodes in patients with superficial carcinoma of the thoracic oesophagus. Br J Surg 1999;86:669-73. [Crossref] [PubMed]
- Ruurda JP, van der Sluis PC, van der Horst S, et al. Robot-assisted minimally invasive esophagectomy for esophageal cancer: A systematic review. J Surg Oncol 2015;112:257-65. [Crossref] [PubMed]
- van Hillegersberg R, Seesing MF, Brenkman HJ, et al. Robot-assisted minimally invasive esophagectomy. German version. Chirurg 2016;87:635-42. [Crossref] [PubMed]
- Kim DJ, Park SY, Lee S, et al. Feasibility of a robot-assisted thoracoscopic lymphadenectomy along the recurrent laryngeal nerves in radical esophagectomy for esophageal squamous carcinoma. Surg Endosc 2014;28:1866-73. [Crossref] [PubMed]
- Zhong D, Zhou Y, Li Y, et al. Intraoperative recurrent laryngeal nerve monitoring: a useful method for patients with esophageal cancer. Dis Esophagus 2014;27:444-51. [Crossref] [PubMed]
- Ikeda Y, Inoue T, Ogawa E, et al. Recurrent laryngeal nerve monitoring during thoracoscopic esophagectomy. World J Surg 2014;38:897-901. [Crossref] [PubMed]
- Hikage M, Kamei T, Nakano T, et al. Impact of routine recurrent laryngeal nerve monitoring in prone esophagectomy with mediastinal lymph node dissection. Surg Endosc 2017;31:2986-96. [PubMed]
- Rino Y, Yukawa N, Sato T, et al. Using NU-KNIT® for hemostasis around recurrent laryngeal nerve during transthoracic esophagectomy with lymphadenectomy for esophageal cancer. BMC Res Notes 2014;7:127. [Crossref] [PubMed]
- Graboyes EM, Bradley JP, Meyers BF, et al. Efficacy and safety of acute injection laryngoplasty for vocal cord paralysis following thoracic surgery. Laryngoscope 2011;121:2406-10. [Crossref] [PubMed]
- Bhattacharyya N, Batirel H, Swanson SJ. Improved outcomes with early vocal fold medialization for vocal fold paralysis after thoracic surgery. Auris Nasus Larynx 2003;30:71-5. [Crossref] [PubMed]