Construction of a cell model of β2-adrenoceptor downregulation
Introduction
The mechanisms of β2-adrenergic receptor (β2AR) downregulation is an intensely researched area for the prevention and treatment of bronchial asthma (1). These studies mainly depend on animal models that reveal the pathogenesis as a whole, while the molecular levels are less detailed (2). Balb/c mice have been widely accepted as an animal model of bronchial asthma (3). However, primary cultured airway smooth muscle cells (ASMCs) may be established as a cell model that can reveal the molecular mechanisms of β2AR downregulation. Nevertheless, primary culture of ASMCs is challenging, and the method for stimulation and dosage of stimuli are difficult to determine. Therefore, no classic cell model of β2AR downregulation has been developed so far (4). In this study, we tried to construct a β2AR downregulation cell model based on the previous studies and others’ experiences (2,5,6), which may allow further study of β2AR downregulation.
Materials and methods
Experimental animals
Six to eight-week-old female Balb/c mice [SCXKL (Shanghai) 2011-0003] of a specific pathogen-free grade were purchased from the Shanghai Animal Center of Chinese Academy of Sciences. Mice were given free access to food and were allowed to adapt to their new surroundings for at least 1 week before experiment. This study was approved by the Animal Ethics Committee of Nantong University.
Equipment and reagents
Inverted phase contrast and laser scanning microscopes (Olympus). Refrigerated air dryer (Eppendorf). A WJ-III carbon dioxide cell culture box (Wuhan Langma Reiter Instrument Company). A PCR amplifier (BIO-RAD). A –80 °C cryogenic refrigerator (Heto Ultra Company). A vertical laminar flow clean work bench (Shanghai Purification Equipment Company). Salbutamol (Sigma). A mouse anti-α-actin monoclonal antibody and SABC-FITC immunohistochemical kit (Wuhan Boster Company). A rabbit anti-mouse β2AR polyclonal antibody, rabbit anti-mouse α-tubulin polyclonal antibody, and goat anti-rabbit IgG-horse radish peroxidase (Santa Cruz Biotechnology). Trizol reagent (Invitrogen). D-Hanks solution, 0.25% trypsin, and high glucose Dulbecco’s modified Eagle’s medium (DMEM; Hyclone). Penicillin Sodium for Injection (North China Pharmaceutical Incorporated Company). Streptomycin Sulfate for Injection (Shandong Ruiyang Pharmaceutical Incorporated Company).
Cell culture and identification
Primary culture of mouse ASMCs
After cervical dislocation, mice were sterilized in 75% alcohol for 2 minutes. Then, the trachea and pulmonary tissue were separated immediately and placed in D-Hanks solution containing double antibiotics (penicillin and streptomycin) at 4 °C under sterile conditions. The tracheal out membrane was removed with ophthalmic tweezers, the trachea was excised lengthwise, and the inner membrane was scraped off gently with a scalpel until the tissue became hyaline. These hyaline tissues were cut into about 1 mm3 pieces and affixed to the bottom of the 25 mL culture bottle equidistantly with a dental probe. The bottle was inverted and 2 mL high glucose DMEM containing 20% fetal bovine serum was added without contacting the tissue. Then, the bottle was incubated at 37 °C with 5% CO2 for 3 hours. When the tissue was almost dry, the bottle was inverted to submerge the tissue, followed by 3 days of culture. Then, the medium volume was increased to 5 mL and changed on day 6. Thereafter, half of the medium volume was changed every 5 days.
As observed under an inverted phase contrast microscope, cells gradually outgrew from the tissues. At 80% confluence, the cells were subcultured. Cells were washed with D-Hanks solution two or three times, and then 2 mL of 0.25% trypsin was added to just cover the cells. After incubation for 5 minutes, most cells separated from the container. Then, 6 mL high glucose DMEM containing 20% fetal bovine serum was added to stop the digestion. The cells were passaged at 1:1, 1:2 and 1:3 ratios. Half medium volumes were changed every 5 days.
Purification of mouse ASMCs
ASMCs were purified by differential adhesion. At 15 minutes after the first passage, only some of the cells were adherent. Unattached cells were transferred to a second bottle. After 15 minutes, unattached cells were transferred to a third bottle. Cells in the third bottle were pure ASMCs.
Identification of mouse ASMCs
ASMC morphology was observed by the size, shape, arrangement, and growth of the cells under an inverted phase contrast microscope. For immunocytochemical staining, passage 3-5 ASMCs were grown on glass slides in a 6-well plate. At 80% confluence, the cells were fixed with 4% paraformaldehyde for 20 minutes. Then, the cells were washed with distilled water three times for 3 minutes each time. Endogenous peroxidase activity was blocked by incubation with 0.6% hydrogen peroxide for 30 minutes, and then the cells were washed with 0.01 mol/L PBS three times for 2 minutes each time. Non-specific antibody binding was blocked by incubation with normal goat serum at room temperature for 20 minutes without washing. Cells were incubated with the mouse anti-α-actin monoclonal antibody at room temperature for 20 minutes, and then washed with 0.01 mol/L PBS three times for 2 minutes each time. Next, the cells were incubated with a biotinylated goat anti-rabbit IgG at room temperature for 20 minutes, and then washed as described above. Finally, the cells were incubated with SABC-FITC for 20 minutes, and then washed with 0.01 mol/L PBS three times for 5 minutes each time. After staining, the cells were observed and photographed under a laser scanning microscope.
Stimulation by salbutamol
Passage 3 or 4 ASMCs in the exponential growth phase were seeded in a 24-well plate at 1×106 cells per well. Cells were randomly divided into two groups: control and salbutamol-treated groups. The salbutamol-treated group was cultured with medium containing 250 ng/mL salbutamol for eight days. The control group was cultured with normal medium.
RT-PCR analysis of β2AR mRNA expression in mouse ASMCs
The sequences of mouse α-tubulin and β2AR genes were obtained from the NCBI database (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Nucleotide). Primers were designed using Primer Premier 5, Dnastar analysis software and on-line BLAST analysis, and synthesized by Shanghai Shenggong Biological Engineering Company as follows: α-tubulin: forward, TTGAGCCAGCCAACCAG; reverse, CACCCTCCACAGAATCCA (458 bp); β2AR: forward, ATCTCCTGAAGGTGCTGT; reverse: GATCCGATCCGGTCTAT (321 bp). Total RNA was extracted using Trizol reagent and reverse transcribed to cDNA with a TaKaRa Reverse Transcription Kit according to the manufacturer’s instructions. For PCR, 5 µL cDNA template was used for each amplification. The thermal cycling conditions were initial denaturation for 5 minutes at 95 °C, followed by 25 cycles of denaturation at 95 °C for 30 seconds, annealing at 60 °C for 40 seconds and extension at 72 °C for 60 seconds, and then a final extension at 72 °C for 10 minutes. PCR products were subjected to 1% agarose gel electrophoresis, observed under an ultraviolet lamp, and photographed with a gel analysis system. Electrophoretic bands were analyzed with grayscale scanning software.
Western blot detection of β2AR protein expression in mouse ASMCs
Cells were collected and lysed in RIPA buffer containing a protease inhibitor on ice for 30 minutes with intermittent agitation. The supernatant was collected, mixed with loading buffer, and boiled for 3-5 minutes. The protein samples were then stored at –20 °C until use. Protein concentration was determined by a BCA Protein Quantify Kit (SNBC) using an Eppendorf Biophotometer.
Protein samples (100 µL) were electrophoresed, and then transferred to a polyvinyl difluoride membrane. After blocking with 5% skim milk powder in 1× TBST at 37 °C for 1 hour, the membrane was washed with TBST, and then incubated with primary antibodies (1:1,000) for 1 hour. Then, the membrane was thoroughly washed with TBST, followed by incubation with the second antibody (1:6,000) for 1 hour. The membrane was washed, and then visualized by ECL reagent. α-tubulin was used as an internal control.
Statistical analysis
Statistical analyses were performed using SPSS software version 16.0. Measurement data are described as . Data with a normal distribution were analyzed using a t-test. A P-value of less than 0.05 was considered to be significant.
Results
Culture and identification of ASMCs
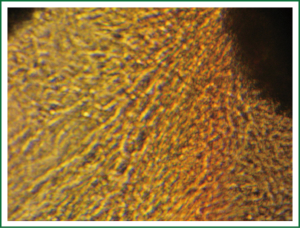
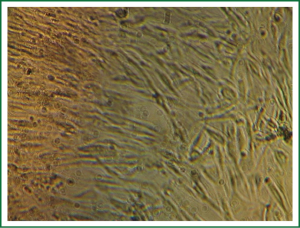
After the tissues were incubated for 3-6 days, a small number of round cells began to migrate and erupt from the outer edges as observed under an inverted phase contrast microscope. In general, 10-15 days was needed for the cells to reach confluency for subculture (Figure 1). During subculture, some tissues separated from the container and would adhere again, followed by outgrowth of cells. The subcultured cells reached confluency in 8-10 days (Figure 2). The growth characteristics of the subcultured cells were similar to those of the primary cells.
ASMC morphology
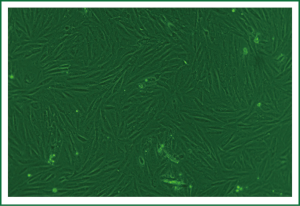
The sizes of primary cultured cells appeared to be different under the inverted phase contrast microscope. Prior to confluency, ASMCs were fusiform, round or polygonal, and had one or multiple nuclei with one or several processes extending toward areas of low cell density. At confluency, ASMCs typically grew as a long fusiform with a ranked fascicular. The cells alternated and overlapped mutually. This typical peak and valley growth is characteristic of smooth muscle cells (Figure 3).
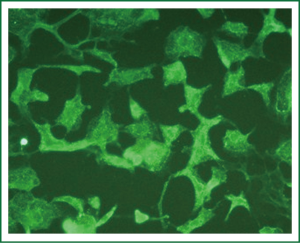
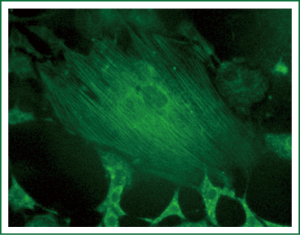
Immunocytochemical staining of α-actin, which is specific for smooth muscle cells, was performed with a mouse anti-α-actin monoclonal antibody. More than 95% of cells were strongly positive for α-actin in the cytoplasm under a laser scanning microscope. α-actin fluorescence was parallel to the longitudinal axis of the cells, indicating smooth muscle α-actin. Some cells were multinucleated (Figures 4,5).
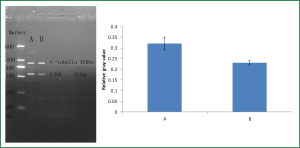
β2AR mRNA expression in mouse ASMCs
β2AR mRNA expression in the salbutamol-treated group was lower than that in the control group as shown by RT-PCR (P<0.05) (Figure 6).
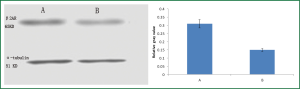
β2AR protein expression in mouse ASMCs
β2AR protein expression in the salbutamol-treated group was lower than that in the control as group as shown by western blotting (P<0.05) (Figure 7).
Discussion
Cell models have become important for the study of various diseases (7). Although there is no uniform standard, a cell model should conform to the following criteria (8): (I) Primary culture and expansion of cells; (II) There should be an appropriate treatment or modification based on the pathogenesis or characteristics of the specific model; (III) A corresponding index or experimental method is needed (an acknowledged gold standard is optimal) to test whether establishment of the cell model is successful or needs improving.
β2AR downregulation is an important aspect of β2AR desensitization (9) that involves numerous factors, and has an uncertain observational indicator and unstable means of testing (10). Therefore, in this study, we constructed a cell model of β2AR downregulation as the observational indicator. As mentioned previously, the present studies of a cell model of β2AR downregulation have been deficient. Vayttaden (4) et al. overexpressed and silenced wild-type β2AR in HEK293 cells by genetic engineering methods to study the molecular mechanisms of β2AR downregulation. However, their study did not use a stimulation factor or airway smooth muscle cells. Thus, it is not a qualified model from the perspective of cell models.
β2AR agonists are effective drugs for clinical treatment of asthma. However, their application is limited because of β2AR downregulation (11). To study the molecular mechanisms of β2AR downregulation, models should include airway smooth muscle from patients or animal models of asthma and adopt a stimulation method similar to the clinical setting during establishment (12). Therefore, construction of a cell model of β2AR downregulation by stimulating the airway smooth muscle of an animal model with salbutamol or another β2AR agonist may reflect the molecular mechanisms of β2AR downregulation. Balb/c mice have been widely accepted as an animal model of bronchial asthma (3). Construction of cell models of β2AR downregulation with airway smooth muscle is direct and objective. Salbutamol is one of the most commonly used short-acting β2AR agonist because of minor systemic side effects, low-cost, and a rapid effect of relieving acute dyspnea (13). Our study adopted salbutamol as the stimulus to construct the cell model, so that it was more similar to the pathological status of clinical patients who are medicated with salbutamol. Thus, our study is more accurate and reliable. We constructed the cell model of β2AR downregulation by adding salbutamol to the medium and stimulating ASMCs over a long-term. The concentration of salbutamol used in this study was determined according to the concentration applied to normal human ASMCs by Luo et al. (14). Their study showed that salbutamol induces apoptosis of human ASMCs in a time- and concentration-dependent manner. Therefore, the stimulation time and concentration of salbutamol needs further exploration during construction of the cell model of β2AR downregulation.
Conclusions
In our study, Balb/c mouse ASMCs were primarily cultured and stimulated by salbutamol. β2AR expression in the control and salbutamol-treated groups was evaluated by RT-PCR and western blot analyses, and together with observation of cytomorphology and identification by immunocytochemistry. We successfully achieved preliminary construction of a cell model of β2AR downregulation. We are currently in the exploration stage, and this cell model requires further optimization such as special conditions other than the short-acting β2AR agonist as well as its optimal concentration. Furthermore, we should explore whether it is feasible to obtain a cell model by primary culture of ASMCs derived from animal models of β2AR downregulation and whether it would be equivalent to the cell model constructed in this study.
Acknowledgements
This work was supported by Natural Science Foundation of China [30971306]; and Six big talent peak in jiangsu province project [the seventh batch, 033]; and Nantong social development project [NO: S2009023]; and Nantong fourth period “226 high-level personnel training project” project.
Disclosure: The authors declare no conflict of interest.
References
- O’Byrne PM, van der Linde J, Cockcroft DW, et al. Prolonged bronchoprotection against inhaled methacholine by inhaled BI 1744, a long-acting beta(2)-agonist, in patients with mild asthma. J Allergy Clin Immunol 2009;124:1217-21. [PubMed]
- Liu H, Zhou LF, Zhang Q, et al. Increased RhoGDI2 and peroxiredoxin 5 levels in asthmatic murine model of beta2-adrenoceptor desensitization: a proteomics approach. Chin Med J (Engl) 2008;121:355-62. [PubMed]
- Yu J, Jang SO, Kim BJ, et al. The Effects of Lactobacillus rhamnosus on the Prevention of Asthma in a Murine Model. Allergy Asthma Immunol Res 2010;2:199-205. [PubMed]
- Vayttaden SJ, Friedman J, Tran TM, et al. Quantitative modeling of GRK-mediated beta2AR regulation. PLoS Comput Biol 2010;6:e1000647. [PubMed]
- Liu H, Ni SS, Cai HQ, et al. Construction of beta-2 Adrenergic Receptor Down-Regulative Asthmatic Model. Chin J Respir Crit Care Med 2010;9:122-7.
- Finney PA, Belvisi MG, Donnelly LE, et al. Albuterol-induced downregulation of Gsalpha accounts for pulmonary beta(2)-adrenoceptor desensitization in vivo. J Clin Invest 2000;106:125-35. [PubMed]
- Sharma SV, Haber DA, Settleman J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat Rev Cancer 2010;10:241-53. [PubMed]
- Tandara AA, Mustoe TA. MMP- and TIMP-secretion by human cutaneous keratinocytes and fibroblasts--impact of coculture and hydration. J Plast Reconstr Aesthet Surg 2011;64:108-16. [PubMed]
- Soh UJ, Dores MR, Chen B, et al. Signal transduction by protease-activated receptors. Br J Pharmacol 2010;160:191-203. [PubMed]
- Nino G, Hu A, Grunstein JS, et al. Mechanism of glucocorticoid protection of airway smooth muscle from proasthmatic effects of long-acting beta2-adrenoceptor agonist exposure. J Allergy Clin Immunol 2010;125:1020-7. [PubMed]
- Black JL, Oliver BG, Roth M. Molecular mechanisms of combination therapy with inhaled corticosteroids and long-acting beta-agonists. Chest 2009;136:1095-100. [PubMed]
- Violin JD, DiPilato LM, Yildirim N, et al. beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem 2008;283:2949-61. [PubMed]
- Baker JG. The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol 2010;160:1048-61. [PubMed]
- Luo Y, Lai W, Xu J. Role of salbutamol in inducing apoptosis of cultured human airway smooth muscle cells. Zhonghua Jie He He Hu Xi Za Zhi 2001;24:219-24. [PubMed]