A 4-year surveillance of antimicrobial resistance patterns of Acinetobacter baumanni in a university-affiliated hospital in China
Introduction
Acinetobactor baumannii (A. baumannii) is an important opportunistic pathogen causing a wide spectrum of nosocomial infections including pneumonia, bacteremia, surgical-site infections, secondary meningitis, and urinary tract infections, involving mostly patients with impaired host defenses (1,2). In recent years, the increasing resistance rate of A. baumannii to a wide range of antibiotics as a result of both intrinsic and acquired mechanisms is causing a serious clinical problem, leading to an increase in morbidity and mortality (3-5). Surveillance is therefore important in providing useful information for physicians in choosing empirical antibiotics. It also helps to address specific resistant issues within a region to identify targeted intervention measures (6,7).
In view of the severity of A. baumannii infections and the implications for empirical antibiotic regimens in the setting of high drug resistant strains, we set out to undertake a retrospective analysis of A. baumannii isolated in our hospital in 4-year period. The changing trend and associated factors of antimicrobial susceptibility were reviewed.
Materials and methods
Bacterial isolates
The study period spanned from January 2008 to December 2011. Bacterial isolates were collected from outpatients and inpatients in the First Affiliated Hospital of Nanjing Medical University, a tertiary-care hospital with 2,261-bed in China. Only the first isolate from each patient was included in this study. All the strains were identified using VITEK-2 (biomérieux, France) and API automated systems (biomérieux, France), supplemented by conventional biochemical tests.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was determined by the Kirby-Bauer Disk Diffusion Agar method as recommended by Clinical and Laboratory Standards Institute guidelines (CLSI) (8). Bacterial isolation disk diffusion testing was performed on Muller-Hinton agar (Oxoid, UK), using disks (Oxoid) of cephalosporins (cefuroxime, ceftazidime, cefotaxime, cefepime), compound agents (cefperazone/sulbactam, piperacillin/tazobactam, amoxycillin/clavulanate), carbapenems (imipenem and meropenem), aminoglycosides (amikacin), monobactams (aztreonam), fluoroquinolones (levofloxacin). Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922 were used as control strains for susceptibility tests.
Data management and statistical analysis
Data of antimicrobial susceptibility testing were extracted from the laboratory information system and converted centrally into a standard format using WHONET 5.4 (WHO, Switzerland), which was used for data management. SPSS 17.0 software (SPSS Inc., USA) was used for statistical analysis. Results, presented as numbers (percentage) for categorical variables, were compared using Pearson’s χ2 test. For small samples, Fisher’s exact test was used to analyze as appropriate. Statistical significance was set at P<0.05.
Results
Changing of A. baumannii isolation rate
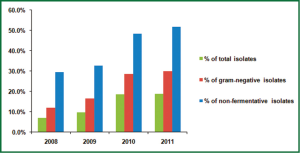
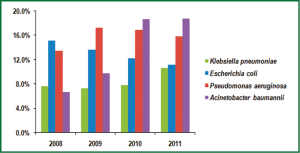
From 2008 to 2011, the cases of bacterial infection in the first affiliated hospital of Nanjing medical university were 4,220, 5,207, 4,851 and 5,960, respectively. Of these pathogens, there was an overall increase in isolation rate of A. baumannii, comprising 7.0%, 9.8%, 18.7% and 18.8% in 2008-2011. The proportion of A. baumannii in gram-negative isolates (from 12.0% in 2008 to 29.9% in 2011) and non-fermentative isolates (from 29.6% to 51.9%) also increased year by year (Figure 1). The top 4 frequently isolated pathogens in gram-negative isolates were shown in Figure 2. Pseudomonas aeruginosa accounted for 13.5% and 17.2% of all isolates in 2008 and 2009, respectively, while A. baumannii became the most common isolated pathogen beyond Pseudomonas aeruginosa in gram-negative isolates from 2010 to 2011.
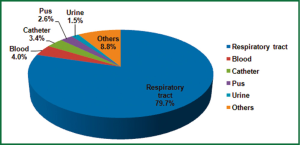
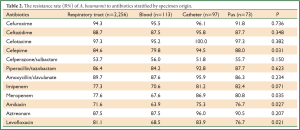
A. baumannii were cultured from respiratory samples (2,256 isolates, 79.7%), followed by blood (113 isolates, 4.0%), catheter (97 isolates, 3.4%), pus (73 isolates, 2.6%), urine (43 isolates, 1.5%) and others (249 isolates, 8.8%) (Figure 3). The main 5 wards that A. baumannii was initially isolated were geriatrics (23.0%), emergency department (17.4%), ICU (16.7%), neurosurgery department (9.6%) and respiratory department (8.3%). The changing trend of A. baumannii distribution stratified by hospital wards from 2008 to 2011 was shown in Figure 4. The greatest burden of A. baumannii infection occurred in geriatric from 2008 to 2011, and only in respiratory department the isolation rate decreased.
Changing trend of antimicrobial susceptibility
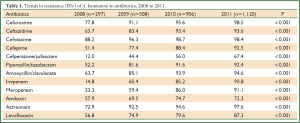
Table 1 showed the resistance rate of A. baumannii to 12 common antimicrobial agents. In general, our study revealed the continuous increase of antimicrobial resistance to all antibiotics studied from 2008 to 2011, and all the changes were statistically significant (P<0.001).
Full Table
The changing trend of resistance rate to cefuroxime, amikacin, aztreonam, levofloxacin, imipenem and meropenem over the 4 years was also shown in Figure 5 to highlight the sharp increase of carbapenems resistance rate. Imipenem resistance rates increased from 14.8% in 2008 to 90.8% in 2011, and meropenem from 23.3% in 2008 to 91.1% in 2011. The resistance rates to other agents increased steadily over the years although fluctuation was observed on amikacin (Table 1, Figure 5).
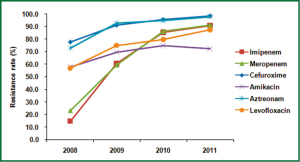
The drug resistance to cephalosporins was also serious during the study period. More than 90% of A. baumannii were resistant to cephalosporins in 2011, with 98.5% resistance rate to cefuroxime and 98.4% resistance rate to cefotaxime. The same increasing trends were also observed in A. baumannii against aminoglycosides, monocyclic β-lactams, quinolones and compound agents. The resistance rate of cefperazone/sulbactam also showed a rapid increase from 12.0% in 2008 to 67.4% in 2011.
Antimicrobial susceptibility stratified by specimen origin
Table 2 summarized the resistance rate of A. baumannii obtained from 4 sites. The resistance rate to cefepime, meropenem, amikacin and levofloxacin significantly differs among A. baumannii from different samples (P-value were 0.031, 0.035, 0.027 and 0.021, respectively). In addition, a relatively lower resistance rate occurred in blood isolates than others, while a higher rate was found in catheter isolates.
Antimicrobial susceptibility stratified by hospital wards
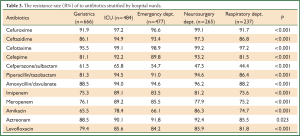
A. baumannii isolated from different wards varied in resistance rate to all antimicrobial agents we studied, and the differences were all significant (P<0.001). Worth noting that the highest resistant rate was observed in neurosurgery department, however, in geriatrics and respiratory department the resistance rates were lower (Table 3).
Full Table
Antimicrobial susceptibility stratified by patients’ gender
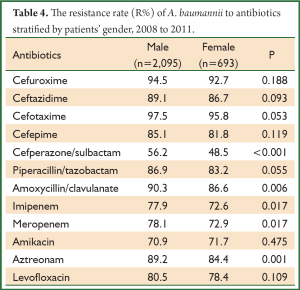
We divided the patients into male and female group to analyze the difference in the level of drug resistance. As listed in Table 4, the resistance rate to cefperazone/sulbactam, amoxycillin/clavulanate, imipenem, meropenem and aztreonam were statistically significant between male and female. Interestingly, the resistance rates to these 5 antibiotics in male group were all higher than female.
Full Table
Antimicrobial susceptibility stratified by patients’ age
We divided patients into 10 groups at intervals of ten-year-old to analyze the drug resistance. Data of group 1 (under ten years old) were unavailable for the number was less than 10. The resistance rates to most antibiotics evaluated were statistically different in 9 age groups except meropenem and aztreonam (Table 5). Moreover, the 11-20 and 21-30 group showed higher resistance rates, and the ≥91 group showed the lowest resistance rates compared with other age groups.
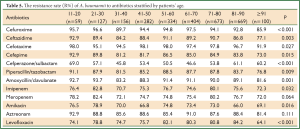
Full Table
Discussion
A. baumannii is an opportunistic pathogen that is frequently involved in a variety of infection including pneumonia, septicaemia and urinary tract infection following hospitalization of patients with more severe illness (9). The ability to chronically colonize patients and cause outbreaks which are usually hard to eradicate poses significant challenge to infection control and increases healthcare expenditure (10).
During the study period, the proportion of A. baumannii in all bacterial isolates had a dramatic increase, from 7.0% in 2008 to 18.8% in 2011, advancing to the most common pathogen in gram-negative isolates, thus garnered significant attention.
In the present study, nearly 80% of A. baumannii recovered from clinical specimens were of respiratory origin. Other sources were blood, catheter, pus and urine. This can be partly explained by the fact that A. baumannii, as the 2th most frequent pathogen causing respiratory tract infection like pneumonia (11), was thus more frequently detected in respiratory sample. In addition, the low examination rate of sterile fluids also resulted in the low isolation rate of A. baumannii in our hospital these years. We recommend clinicians to pay more attention to the detection value of A. baumannii in sterile fluids, so as to increase the examination rate of body fluids like blood, perfusate and serous effusion.
Our data showed that patients with A. baumannii infection mainly came from geriatrics, emergency department, ICU, neurosurgery department and respiratory department. And geriatrics remained the highest isolation rate for 4 years. It may be because patients in these sections were all in critical condition, long duration of hospitalization, lower immune defense, suffering from severe underlying diseases or frequent invasive procedures like tracheotomy. Therefore, patients were in high-risk of hospital-acquired infection (12). In nearly all wards in China, many patients usually resident in one room, so adequate patient separation and infection control measures are important to reduce nosocomial infection.
Our study revealed that there had been a continuous increase in the in vitro resistance to 12 antimicrobial agents we evaluated, including cephalosporins, carbapenems, aminoglycoside, quinolones and compound agents. The striking increase in resistance to carbapenems is of particular interest, since this class of antimicrobial agent was, until recently, considered to be among the most potent against many microorganisms including A. baumannii. The resistance rates to carbapenems rapidly increased throughout the study period, for imipenem from 14.8% in 2008 to 90.8% in 2011, and for meropenem from 23.3% in 2008 to 91.1% in 2011. The changing trend was much more obvious than other reports showing carbapenems-resistant growth. In Taiwan, the prevalence of imipenem-resistance over 10 years was from 3.4% in 2002 to 58.7% in 2010 (13). A SENTRY study also reported that resistance to imipenem changed from 34.5% in 2006 to 59.8% in 2009 overall worldwide (14). The major mechanism of carbapenems resistance in A. baumannii is the production of carbapenemases including Ambler class B metallo-β-lactamases (MBLs) and the carbapenem-hydrolyzing class D β-lactamases (CHDLs), also called oxacillinases (OXAs), with the latter more frequently in A. baumannii. Several studies in China have showed that blaoxa-23-like gene, perfectly associated with carbapenem resistance, could be used to predict imipenem resistance of A. baumannii (15,16).
The increasing emergent of highly aminoglycosides-resistant strains caused a major concern. Our result on resistance rate to amikacin was 94.6% in 2011 higher than 34% in Latin America reported by a SENTRY antimicrobial surveillance program (17). Moreover, the resistance rates to β-lactams antibiotics such as third/fourth-generation cephalosporins or compound agents increased gradually, especially for cefperazone/sulbactam, from 12.0% in 2008 to 67.4% in 2011.
It seems that our resistance rates of A. baumannii to antibiotics were generally much higher than data from other regions. The difference could likely be due to inappropriate, uncontrolled empiric therapy or cross acquisition of resistance in our hospital. These reasons highlight the urgent need for supervising antibiotic use and strengthening infection control measures in our hospital. New therapeutic strategy should be introduced in clinical practice for the treatment of multi-resistant A. baumannii infection. Recent observations had shown that combination antibiotic therapy (e.g., polymyxin B combined with imipenem, imipenem plus rifampin, or the triple combination of poly-myxin B, imipenem and rifampin) was synergistic when tested against imipenem-resistant strains (18).
Although increasing resistance rate of A. baumannii worldwide has been reported before (19,20), few studies reported antimicrobial susceptibility patterns affected by several independent factors: specimen origin, hospital wards, patients’ gender and age.
As to sample origin, the resistance rate to cefepime, meropenem, amikacin and levofloxacin significantly differs. The isolates from blood showed the lowest resistance rate. In contrast, the highest resistance rate was found in catheter, which may be related to nosocomial infection. At least 37.5% of all nosocomial infections were due to cross-transmissions of colonized pathogen between patients, thus increase the risk of drug-resistance (21). Although A. baumannii was mostly detected in respiratory samples, the resistance rate was relatively not so high. Sample size might be the reason, which was 2,256 from respiratory tract, twenty-fold greater than others.
Data concerning the resistance rate of A. baumannii from patients receiving care in different wards were also analyzed. In geriatrics and respiratory department, the resistance rates of A. baumannii were much lower. Of interest, the highest resistance rate was found in patients from neurosurgery department rather than ICU as other studies reported (22,23). Characteristics of neurosurgery, such as a higher proportion of patients undergoing neurosurgical surgery, a more frequent use of invasive procedures like intraventricular catheters, and a more illegitimate antibiotics use because of the unremarkable clinical manifestations of post-surgical infections, compared to other hospital wards, may cause the result (24).
We analyzed the resistance rates according to patients’ gender and age. The statistically difference was only found in 5 antibiotics between male and female, but it is still noteworthy that the resistance rate in male group were all higher than female in these 5 antibiotics. To our knowledge, variation in resistance rate profiles among different age groups has rarely been addressed. Our study revealed that the resistance rates to most of antibiotics were statistically significant among 9 age groups. The 11-20 and 21-30 age groups showed higher resistance rates compared to other age groups. The lowest resistance rate was in ≥91 age group, which was coincidently in agreement with the lower resistance rate in geriatrics. It seems that elderly patients (≥91) were more susceptible to antimicrobial agents during the therapy of A. baumannii infections. Whether the lower drug-resistance of isolates from elderly patients resulted from the rational use of antibiotics or less frequency of clonal transmission remained to be elucidated.
It should be acknowledged that our data were retrieved from only one hospital, so continuous multi-center surveillance of antimicrobial susceptibility of A. baumannii in China is still necessary to generate enough representative data. Moreover, molecular typing analysis, such as pulsed-field gel electrophoresis (PFGE), multilocus sequence typing (MLST), and drug-resistance gene typing (DRGT), should be performed. Thus, existing outbreaks caused by epidemic clonal spread of a single resistant strain could be monitored.
In conclusion, this study investigated antimicrobial susceptibility of A. baumannii and its related influencing factors in a university-affiliated hospital in China during 4 years. Knowing the rapidly increased resistance rate of A. baumannii and the association with specimen origin, hospital wards, patients’ gender and age, is important to better understanding the antimicrobial patterns, and optimize antimicrobial prescription policies to control the occurrence of drug-resistant A. baumannii.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81000754) and a grant from the Key Laboratory for Laboratory Medicine of Jiangsu Province of China (No. XK201114).
Disclosure: The authors declare no conflict of interest.
References
- Bassetti M, Righi E, Esposito S, et al. Drug treatment for multidrug-resistant Acinetobacter baumannii infections. Future Microbiol 2008;3:649-60. [PubMed]
- Wang H, Guo P, Sun H, et al. Molecular epidemiology of clinical isolates of carbapenem-resistant Acinetobacter spp. from Chinese hospitals. Antimicrob Agents Chemother 2007;51:4022-8. [PubMed]
- Sunenshine RH, Wright MO, Maragakis LL, et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 2007;13:97-103. [PubMed]
- Falagas ME, Bliziotis IA, Siempos II. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care 2006;10:R48. [PubMed]
- Jung JY, Park MS, Kim SE, et al. Risk factors for multi-drug resistant Acinetobacter baumannii bacteremia in patients with colonization in the intensive care unit. BMC Infect Dis 2010;10:228. [PubMed]
- Lauderdale TL, Clifford McDonald L, Shiau YR, et al. The status of antimicrobial resistance in Taiwan among gram-negative pathogens: the Taiwan surveillance of antimicrobial resistance (TSAR) program, 2000. Diagn Microbiol Infect Dis 2004;48:211-9. [PubMed]
- White AR, BSAC Working Parties on Resistance Surveillance. The British Society for Antimicrobial Chemotherapy Resistance Surveillance Project: a successful collaborative model. J Antimicrob Chemother 2008;62 Suppl 2:ii3-14. [PubMed]
- Clinical and Laboratory Standards Institute. eds. Analysis and presentation of cumulative susceptibility test data; approved guideline, 2nd ed. Wayne: Clinical and Laboratory Standards Institute, 2006.
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008;21:538-82. [PubMed]
- Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 2007;5:939-51. [PubMed]
- Xia W, Chen Y, Mei Y, et al. Changing trend of antimicrobial resistance among pathogens isolated from lower respiratory tract at a university-affiliated hospital of China, 2006-2010. J Thorac Dis 2012;4:284-91. [PubMed]
- Mireya UA, Martí PO, Xavier KV, et al. Nosocomial infections in paediatric and neonatal intensive care units. J Infect 2007;54:212-20. [PubMed]
- Kuo SC, Chang SC, Wang HY, et al. Emergence of extensively drug-resistant Acinetobacter baumannii complex over 10 years: nationwide data from the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program. BMC Infect Dis 2012;12:200. [PubMed]
- Gales AC, Jones RN, Sader HS. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program (2006-09). J Antimicrob Chemother 2011;66:2070-4. [PubMed]
- Dai W, Huang S, Sun S, et al. Nosocomial spread of carbapenem-resistant Acinetobacter baumannii (types ST75 and ST137) carrying blaOXA-23-like gene with an upstream ISAba1 in a Chinese hospital. Infect Genet Evol 2013;14:98-101. [PubMed]
- Hu Q, Hu Z, Li J, et al. Detection of OXA-type carbapenemases and integrons among carbapenem-resistant Acinetobactor baumannii in a teaching hospital in China. J Basic Microbiol 2011;51:467-72. [PubMed]
- Tognim MC, Andrade SS, Silbert S, et al. Resistance trends of Acinetobacter spp. in Latin America and characterization of international dissemination of multi-drug resistant strains: five-year report of the SENTRY Antimicrobial Surveillance Program. Int J Infect Dis 2004;8:284-91. [PubMed]
- Yoon J, Urban C, Terzian C, et al. In vitro double and triple synergistic activities of Polymyxin B, imipenem, and rifampin against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2004;48:753-7. [PubMed]
- Park YK, Jung SI, Park KH, et al. Changes in antimicrobial susceptibility and major clones of Acinetobacter calcoaceticus-baumannii complex isolates from a single hospital in Korea over 7 years. J Med Microbiol 2012;61:71-9. [PubMed]
- Neonakis IK, Spandidos DA, Petinaki E. Confronting multidrug-resistant Acinetobacter baumannii: a review. Int J Antimicrob Agents 2011;37:102-9. [PubMed]
- Kuo LC, Yu CJ, Kuo ML, et al. Antimicrobial resistance of bacterial isolates from respiratory care wards in Taiwan: a horizontal surveillance study. Int J Antimicrob Agents 2008;31:420-6. [PubMed]
- Wadl M, Heckenbach K, Noll I, et al. Increasing occurrence of multidrug-resistance in Acinetobacter baumannii isolates from four German University Hospitals, 2002-2006. Infection 2010;38:47-51. [PubMed]
- Sunenshine RH, Wright MO, Maragakis LL, et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 2007;13:97-103. [PubMed]
- Rodríguez Guardado A, Blanco A, Asensi V, et al. Multidrug-resistant Acinetobacter meningitis in neurosurgical patients with intraventricular catheters: assessment of different treatments. J Antimicrob Chemother 2008;61:908-13. [PubMed]