The role of indacaterol for chronic obstructive pulmonary disease (COPD)
Introduction
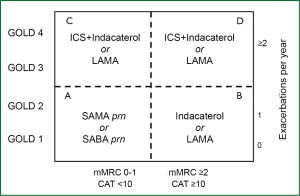
Inhaled bronchodilators are the mainstay of the current management of chronic obstructive pulmonary disease (COPD) (1). Only for subjects that can be classified as group A patients according to the last GOLD classification of severity because they have few symptoms and a low risk of exacerbations, a short-acting bronchodilator is recommended as first choice (2). For all other COPD patients long-acting formulations are preferred over short-acting formulations (2). Two classes of long-acting inhaled bronchodilators are available—long-acting β2-agonists (LABAs) and long-acting muscarinic antagonists (LAMAs). LABAs directly induce bronchodilation by relaxing airway smooth muscle through stimulation of β2-adrenoceptors, whereas LAMAs prevent acetylcholine-induced bronchoconstriction by acting as competitive antagonists on muscarinic receptors (3).
Because of the central role of long-acting bronchodilators in the treatment of COPD, in recent years there has been a renewed interest in the field and now once-daily (OD) bronchodilators are in development in an attempt to simplify the management of COPD patients (3,4). In effect, an important step in simplifying COPD management and improving adherence with prescribed therapy is to reduce the dose frequency (3,4). Therefore, the incorporation of OD dosing is an important strategy to improve compliance, and is a regime preferred by most patients (3,4). Indacaterol is the first ultra-LABA approved that has a 24-hour bronchodilatory effect, allowing for OD administration (5).
Pharmacological profile of indacaterol
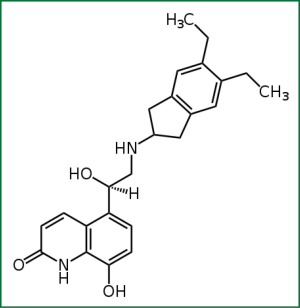
Indacaterol is a novel chirally pure inhaled ultra-LABA. Within a series of 8-hydroxyquinoline 2-aminoindan derived β2-adrenoceptor agonists, lipophilicity was used as the basis for the design and rationalization of their onset and duration of action profiles, as assessed by a guinea pig tracheal-strip assay. In addition to lipophilicity, potency and intrinsic efficacy have also been shown to be contributing factors in regulating these in vitro time course profiles. Selected from these studies was the 5,6-diethyl substituted indan analogue, (R)-5-{2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl}-8-hydroxyquinolin-2(1H)-one, indacaterol (Figure 1) (6). Extensive preclinical studies involving indacaterol have been performed both in vitro and in vivo and have documented that it demonstrates a unique rapid onset of action and a bronchodilating effect that lasts for 24 h (5,6).
Indacaterol seems to have a high intrinsic activity at human β2-adrenoceptors in vitro (Table 1). The mean maximum effect (Emax) for indacaterol was 73% of the maximum effect of isoprenaline, compared with 90%, 38%, and 47% for formoterol, salmeterol, and salbutamol, respectively (7). Like formoterol, indacaterol is a very weak agonist at the β1-adrenoceptor (mean Emax =16% of the maximal effect of isoprenaline) but acts as a full agonist at the β3-adrenoceptor (mean Emax =113%) (7). Studies with isolated human bronchi and small-airway lung slices showed that indacaterol behaves as a high efficacy β2-agonist, with an onset of action that is not significantly different from that of formoterol or salbutamol but significantly faster than that of salmeterol, and a significantly longer duration of action than either formoterol or salmeterol (8,9). In particular, a study that compared the properties of indacaterol with those of salmeterol, formoterol, and salbutamol on small airways in precision-cut human lung slices contracted with carbachol (9) confirmed that the onset of action is fast for salbutamol, formoterol, and indacaterol, whereas it is significantly slower for salmeterol, and indicated that indacaterol and formoterol have a higher intrinsic efficacy than salbutamol and salmeterol. It was also shown that indacaterol, in contrast to salmeterol, does not antagonize the bronchorelaxant effect of a short-acting β2-agonist (8).

Full Table
It is noteworthy that no tachyphylaxis has been demonstrated for indacaterol, although significant improvement in protection against 5-hydroxytryptamine-induced bronchoconstriction has been documented after 5-day dosing of indacaterol and formoterol (compared with a single treatment), but not with salmeterol, at least in guinea pig airways (7). The fact that indacaterol behaves as a nearly full β2-agonist could explain why indacaterol does not induce tachyphylaxis and also does not antagonize the bronchorelaxant effect of a short-acting β2-agonist. Although low-efficacy agonists may cause less receptor desensitization at equal occupancy, they require more receptors to generate a subsequent response and so will be more sensitive to loss of functional receptors (10). High-efficacy agonists, in contrast, may cause a greater loss of receptors, but are more tolerant to this, as they have “spare receptors,” resulting in a loss in potency but not necessarily any loss of maximal effect and are therefore less sensitive to loss of receptors through desensitization (10). Preclinical data also suggest that, for a given degree of bronchodilator activity, indacaterol has a greater cardiovascular safety margin than formoterol or salmeterol (7).
The faster onset of action and longer duration of action of indacaterol compared with some other β2-agonists may be related to interactions with lipid bilayers (11). Indacaterol and salmeterol show no major, but several minor, differences in their steady-state and kinetic interactions with lipid membranes, and the sum of these small differences, including higher partitioning of indacaterol into the microenvironment of the receptor and its faster membrane permeation, is thought to contribute to its faster onset and longer duration of therapeutic action. A striking difference was observed between indacaterol and salmeterol on membrane fluidity. Although indacaterol did not alter membrane fluidity, salmeterol drastically increased membrane fluidity. This may affect the function of the β2-adrenoceptor, reducing the intrinsic efficacy of salmeterol (11). It has also been suggested that lipid rafts, which are areas of cell membranes in which β2-adrenoceptors are held together in close contact with signaling molecules and effectors, and calveolae, which are a special type of lipid raft, being small (50-100 nm) invaginations of the plasma membrane in airway smooth muscle, might play a role in the long duration of action of indacaterol (11). Indacaterol has a 2-fold higher affinity for raft microdomains compared with salmeterol, which might contribute to the difference in duration of action between these two drugs. It has also been suggested that the higher intrinsic efficacy of indacaterol offsets the high lipophilicity that is important for achieving the long duration of action (12). In fact, in primary human bronchial smooth muscle cells, indacaterol displays a similar intrinsic activity to formoterol that, combined with comparable lipophilicity, translates to a faster rate of cAMP accumulation, which plays a key role in β2-adrenoceptor-induced smooth muscle relaxation in the airways.
Clinical development of indacaterol
Pharmacokinetics and dose-finding
(14C) indacaterol single 800-μg (free base) dose administered orally to healthy male subjects was absorbed fairly rapidly with Cmax occurring at 1.75 h (13). Unmodified indacaterol was the major circulating drug-related component, with the monohydroxylated metabolite, P26.9, its glucuronide, P19, and the 8-O-glucuronide of indacaterol, P37, also contributing significantly to the serum profile. Excretion of radioactivity and indacaterol into urine accounted for: 10% and 0.55% of the dose, respectively. Excretion of radioactivity, indacaterol, and P26.9 + P30.3 into feces accounted for: 85%, 55%, and 24% of the dose, respectively. No ethnicity effect was observed on indacaterol systemic pharmacokinetic profile (14).
Preliminary studies documented that, after inhalation, indacaterol is rapidly absorbed into the systemic circulation with a median Tmax of 15 min. It has linear and dose-proportional pharmacokinetics, and steady state is reached within 12 days of OD dosing at doses of 150, 300 and 600 µg (15).
In patients with COPD, comprehensive assessment of the dose response relationship of indacaterol provided a robust confirmation that 75 μg is the minimum effective dose, and that 150 and 300 μg provided optimal bronchodilation, particularly in patients with severe disease (16). It is reasonable and safe to increase the dose of indacaterol in those stable COPD patients who are under regular therapy with indacaterol 150 μg from which they do not draw the maximum benefit because they are unable to perceive bronchodilation. However, only a minority of patients seem to benefit from this dose escalation, at least in terms of spirometric improvement (17).
Short-term studies
Several short-term studies have explored the effect of indacaterol in COPD patients. Single doses (150 and 300 μg) of indacaterol demonstrated a fast onset of action similar to that for salbutamol and faster than that for salmeterol-fluticasone (18). Moreover, OD indacaterol (150 μg) is at least as effective as tiotropium bromide, with a faster onset of action (within 5 min) (19) and a slightly superior activity in reducing lung hyperinflation (20) on the first day of dosing.
Indacaterol 300 μg resulted in significant improvement in exercise endurance time, not only after 3 weeks but also after a single-dose (21). Reduced hyperinflation was suggested to be one of the reasons for improved exercise performance achieved following indacaterol treatment (21). In any case, there is evidence that indacaterol 150 μg improves daily physical activity in patients with COPD. In a Japanese study (22), the number of steps, duration of moderate or greater physical activity, and energy expenditure were significantly increased after treatment with indacaterol compared with baseline data in all patients with COPD; the metabolic equivalent of task was also significantly enhanced after treatment with indacaterol.
It has also been documented that a regular treatment with indacaterol does not alter bronchodilator response to repeated doses of this short-acting β2-agonist in COPD patients (23).
Long-term studies
The efficacy of indacaterol in the maintenance treatment of adults with COPD has been assessed in large, randomized, double-blind, parallel-group, placebo-controlled, multicenter phase III trials (24-31).
Analysis of these trials (32) shows that 150 and/or 300 μg of indacaterol OD was more effective than tiotropium bromide, formoterol, or salmeterol for improving trough FEV1 values versus placebo. COPD exacerbations were significantly reduced versus placebo for 150 or 300 μg of indacaterol OD. In a 52-week study (24), OD treatment with indacaterol prolonged the time to the first COPD exacerbation and was effective in reducing incidence and frequency of COPD exacerbations, with no significant difference between indacaterol and formoterol. Patients treated with indacaterol had a significantly higher percentage of days with no use of as-needed rescue salbutamol than did placebo recipients in all large studies. Moreover, the percentages of days with no rescue medication were significantly (P<0.05) higher in the indacaterol groups than the active comparator groups in all studies. In general, indacaterol seemed to have greater effects on most COPD symptoms than tiotropium bromide, formoterol, or salmeterol, although differences between indacaterol and active comparators were not consistently statistically significant. Indacaterol also provided significant and clinically relevant better health-related quality of life. In all studies designed to investigate whether indacaterol has the same tolerability of LABAs already on the market, indacaterol was well tolerated at all doses and with a good overall safety profile (5).
A Bayesian mixed treatment comparison that used individual patient-level data from four randomized controlled trials concluded that indacaterol is expected to be comparable to formoterol, salmeterol, and tiotropium, providing higher FEV1 than formoterol and salmeterol and greater improvement in the St. George’s Respiratory Questionnaire (SGRQ) total score than tiotropium. Indacaterol 150 μg provided comparable improvement in dyspnea, while indacaterol 300 μg demonstrated the greatest response overall (33).
Since dyspnea is the most troublesome symptom of COPD, and is often frightening for the patient, it has been examined in focused systematic reviews and meta-analyses to establish the real importance of indacaterol in the maintenance treatment of COPD.
A systematic review and meta-analysis of available randomized placebo-controlled studies used number of patients achieving the minimum clinically important difference (MCID) for Transition dyspnea index (TDI) score ≥1 as an outcome measure, and evaluated the efficacy of OD indacaterol on TDI scores in patients with stable COPD (34). A favorable effect was consistently obtained for indacaterol over placebo. A trend of increasing patient benefit was observed as indacaterol doses increased. In effect, the results of a post-hoc analysis of pooled data from clinical studies involving 3,177 patients with COPD suggest that indacaterol 300 μg may be a useful treatment option for subjects who experience more severe breathlessness, those with ‘more dyspnoea’ [modified Medical Research Council (Mmrc) scale ≥2] (35). Intriguingly, an ample proportion of patients respond with a MCID from baseline in TDI total score also after indacaterol 75 μg, with broadly similar effects in subgroups of patients with moderate COPD (GOLD II) or with severe-to-very severe COPD (GOLD III-IV) (36).
Many of the pivotal placebo-controlled studies with indacaterol included pre-specified analyses of the primary efficacy outcome (trough FEV1 after 12 weeks of treatment) in patient subgroups defined according to factors such as COPD severity, use of inhaled corticosteroids (ICS), age and smoking status. A post-hoc analysis of pooled clinical study data that investigated efficacy and safety of indacaterol compared with placebo and other was reviewed using pooled 6-month data from four clinical studies with 3,035 patients treated with indacaterol and the twice-daily LABAs (37). Although many patients had pre-existing cerebro- and cardiovascular conditions, there was no significant increase in the risk for cerebro- and cardiovascular adverse events with indacaterol compared with placebo, nor with formoterol or salmeterol. Neither the incidence nor the relative risk increased numerically with increasing dose of indacaterol. Electrocardiogram measurements of QTc interval were also reported, since QTc interval prolongation is an indication of possible arrhythmogenic long-acting bronchodilators (formoterol, salmeterol, open-label tiotropium) in patient subgroups defined by COPD severity and ICS use at baseline, showed that indacaterol maintained its efficacy regardless of disease severity or use of concurrent ICS. Hazard ratios versus placebo for time to first COPD exacerbation showed a significant effect of indacaterol in nonusers [150 µg, 0.47 (P=0.001); 300 µg, 0.64 (P<0.05)] and a smaller nonsignificant effect in ICS users (0.77 and 0.72 for 150 and 300 µg, respectively). Indacaterol 150 μg had the best overall efficacy profile in the GOLD stage II patients while, in patients with more severe disease, indacaterol 300 μg provided useful improvements in dyspnoea (38). Indacaterol provided effective bronchodilation with significant, clinically relevant improvements in dyspnoea and health status compared with placebo also when it was given to patients with moderate-to-severe COPD not receiving other maintenance treatments (39).
Safety
In all studies, indacaterol was well tolerated at all doses and with a good overall safety profile. The rates of adverse events characteristic of β2-agonist, including muscle spasm, headache, and tremor, were comparable between indacaterol and placebo (5).
The cerebro- and cardiovascular safety of indacaterol effects. With all the LABAs, the incidence of notable values was low and similar to placebo. Increases of >60 ms occurred in 0.1-0.3% of patients receiving indacaterol and 0.3% of placebo patients.
An analysis of major cardiovascular adverse events (MACEs) with indacaterol and the twice-daily LABAs, using pooled data from all studies of ≥12 weeks’ duration, reported a nonsignificant reduction with all LABAs relative to placebo (40). There was no relationship with indacaterol dose. Interestingly, also results for QTc interval, plasma potassium, and blood glucose showed no clinically significant changes with indacaterol treatment.
In some trials, cough was the most common adverse event, with the mean percentage of attended visits at which patients experienced cough after inhalation of indacaterol that ranged from 14.1% to 18.4% across the indacaterol dose groups, compared with 2% in the placebo group, and increased slightly with increasing indacaterol dose (40). For the majority of patients, the cough started within 15 seconds of inhalation and lasted for ≤15 seconds; the median duration in the indacaterol groups at each visit was ≤6 seconds.
Cost-effectiveness
The majority of economic evaluations have indicated that pharmacotherapy for COPD in ambulatory care is cost-effective. Cost-effectiveness seems to derive from an improvement in lung function and a reduction in the number of exacerbations, which translates into cost savings from fewer hospitalizations. These cost savings then need to be contrasted with the price of pharmacotherapy (41).
A cost-utility analysis showed that indacaterol 150 μg is dominant (lower total costs and better outcomes) against tiotropium and salmeterol (42). An alternative analysis comparing indacaterol 300 μg (maximum dose) to tiotropium in Germany showed an incremental cost-effectiveness ratio (ICER) of approximately EUR 28,300 per quality-adjusted life year (QALY) (43). Also in UK Indacaterol dominated in the comparison with salmeterol producing an incremental QALY gain of 0.008 and cost savings of £ 110 per patient over a 3-year time horizon (44). In the comparison with tiotropium over the same time horizon, indacaterol remained the dominant strategy, producing an incremental QALY gain of 0.008 and cost savings of £ 248 per patient. The one-way sensitivity analysis indicated that the proportion of patients in each of the COPD stages and the mortality rate associated with very severe COPD were the variables with the largest impact on the results. The probabilistic sensitivity analyses showed that over 72% and 89% of the iterations when compared with salmeterol and tiotropium, respectively, produced dominant results for indacaterol.
Positioning indacaterol in the therapeutic scheme of COPD
When we treat a patient with mild/moderate stable COPD, we must always question whether it is better to start with a β-agonist or an antimuscarinic agent and if the answer is yes, as we believe that it is right, we must also ask whether it is appropriate to put all patients with COPD into regular treatment with a long-lasting bronchodilator (45).
Current guidelines do not distinguish between the efficacy of bronchodilators and suggest that the choice between them depends on availability and patient response in terms of symptom relief and side effects (1,2). However, data from efficacy trials suggest that twice-daily LABAs (salmeterol and formoterol) are preferable to short-acting antimuscarinic agents (ipratropium) (46,47), whereas OD tiotropium, a LAMA (48,49), and indacaterol, an ultra-LABA (50), are superior to LABAs.
A recent systematic review that explored the efficacy and safety of indacaterol in comparison with tiotropium found similar efficacy between indacaterol (150-300 μg/day) and tiotropium (18 μg/day) on trough FEV1 after 12-26 weeks of treatment, but it produced statistically significantly better results in clinical outcomes of dyspnea, use of as needed salbutamol and health status compared with tiotropium (42). Moreover, as already mentioned, a cost-utility analysis showed that indacaterol 150 μg is dominant (lower total costs and better outcomes) against tiotropium and salmeterol (42).
These findings suggest that it is preferable to initiate the treatment of the patient with mild/moderate stable COPD choosing indacaterol (Figure 2). However, in people with COPD who remain symptomatic on treatment with indacaterol, adding a LAMA is the preferable option (51). Concurrent administration of indacaterol and tiotropium is an effective treatment strategy for patients with moderate to severe COPD to promote bronchodilation and lung deflation with no additional safety signal (52). The novel, OD, dual bronchodilator QVA149, containing a fixed dose of the LABA indacaterol with the LAMA glycopyrronium, is under development. In a 26-week trial, QVA149 demonstrated superiority versus treatment with indacaterol or glycopyrronium, with a safety and tolerability profile similar to placebo (53).
Obviously, we must question if and when we must add an ICS (“combined” therapy) or even if it is not preferable to use an ICS/LABA fixed dose combination.
A meta-analysis of 15 placebo-controlled randomized clinical trials suggests that indacaterol monotherapy (150 and 300 μg) is at least as good as formoterol/budesonide (9/320 and 9/160 μg) and comparable with salmeterol/fluticasone (50/250 and 50/500 μg) with respect to lung function (trough FEV1) (54). Indacaterol monotherapy (150 and 300 μg) also provides comparable efficacy in terms of health status (SGRQ total score) versus formoterol/budesonide (9/320 and 9/160 μg) and salmeterol/fluticasone 50/500 μg, as well as improvements in breathlessness (TDI total score) similar to those provided by salmeterol/fluticasone (50/250 and 50/500 μg) (54). It must be highlighted that QVA149 is a potential future treatment option for non-exacerbating symptomatic COPD patients, offering additional benefits over LABA/ICS combinations in terms of significantly superior and clinically relevant improvements in lung function and improvements in important patient-reported outcomes including dyspnoea and rescue medication use (55).
In any case, although it seems that ICSs provide no additional value in reducing exacerbations when used concurrently with LABAs, and this supports the conviction of using indacaterol monotherapy in patients with mild/moderate stable COPD, combination treatment appears to be more effective than a β-agonist alone in patients with low FEV1 (56). In particular, although results from large Phase III trials indicate that QVA149 is able to prevent moderate to severe COPD exacerbations, with an effect that is superior compared with the single LAMA glycopyrronium (57), it is advisable to combine indacaterol with a OD ICS, as mometasone furoate or ciclesonide, in these patients and, in those patients who have many symptoms and a high risk of exacerbations, to combine QVA149 with a OD ICS.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- O’Reilly J, Jones MM, Parnham J, et al. Management of stable chronic obstructive pulmonary disease in primary and secondary care: summary of updated NICE guidance. BMJ 2010;340:c3134. [PubMed]
- Global strategy for the diagnosis, management, and prevention of COPD. Available online: http://www.goldcopd.org/, accessed July 18, 2013.
- Cazzola M, Page CP, Calzetta L, et al. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev 2012;64:450-504. [PubMed]
- Matera MG, Page CP, Cazzola M. Novel bronchodilators for the treatment of chronic obstructive pulmonary disease. Trends Pharmacol Sci 2011;32:495-506. [PubMed]
- Cazzola M, Proietto A, Matera MG. Indacaterol for chronic obstructive pulmonary disease (COPD). Drugs Today (Barc) 2010;46:139-50. [PubMed]
- Baur F, Beattie D, Beer D, et al. The identification of indacaterol as an ultralong-acting inhaled β2-adrenoceptor agonist. J Med Chem 2010;53:3675-84. [PubMed]
- Battram C, Charlton SJ, Cuenoud B, et al. In vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1H-quinolin-2-one (indacaterol), a novel inhaled β2 adrenoceptor agonist with a 24-h duration of action. J Pharmacol Exp Ther 2006;317:762-70. [PubMed]
- Naline E, Trifilieff A, Fairhurst RA, et al. Effect of indacaterol, a novel long acting β2-agonist, on isolated human bronchi. Eur Respir J 2007;29:575-81. [PubMed]
- Sturton RG, Trifilieff A, Nicholson AG, et al. Pharmacological characterization of indacaterol, a novel once daily inhaled β2 adrenoceptor agonist, on small airways in human and rat precision-cut lung slices. J Pharmacol Exp Ther 2008;324:270-5. [PubMed]
- Charlton SJ. Agonist efficacy and receptor desensitization: from partial truths to a fuller picture. Br J Pharmacol 2009;158:165-8. [PubMed]
- Lombardi D, Cuenoud B, Krämer SD. Lipid membrane interactions of indacaterol and salmeterol: do they influence their pharmacological properties? Eur J Pharm Sci 2009;38:533-47. [PubMed]
- Rosethorne EM, Turner RJ, Fairhurst RA, et al. Efficacy is a contributing factor to the clinical onset of bronchodilation of inhaled β2-adrenoceptor agonists. Naunyn Schmiedebergs Arch Pharmacol 2010;382:255-63. [PubMed]
- Kagan M, Dain J, Peng L, et al. Metabolism and pharmacokinetics of indacaterol in humans. Drug Metab Dispos 2012;40:1712-22. [PubMed]
- Matsushima S, Matthews I, Woessner R, et al. Systemic pharmacokinetics of indacaterol, an inhaled once-daily long-acting β2-agonist, in different ethnic populations. Int J Clin Pharmacol Ther 2012;50:545-56. [PubMed]
- Perry S, Woessner R, Kaiser G, et al. Pharmacokinetics of indacaterol after single and multiple inhaled doses. Am J Respir Crit Care Med 2010;181:A4420.
- Renard D, Looby M, Kramer B, et al. Characterization of the bronchodilatory dose response to indacaterol in patients with chronic obstructive pulmonary disease using model-based approaches. Respir Res 2011;12:54. [PubMed]
- Cazzola M, Segreti A, Stirpe E, et al. Effect of an additional dose of indacaterol in COPD patients under regular treatment with indacaterol. Respir Med 2013;107:107-11. [PubMed]
- Balint B, Watz H, Amos C, et al. Onset of action of indacaterol in patients with COPD: comparison with salbutamol and salmeterol-fluticasone. Int J Chron Obstruct Pulmon Dis 2010;5:311-8. [PubMed]
- Vogelmeier C, Ramos-Barbon D, Jack D, et al. Indacaterol provides 24-hour bronchodilation in COPD: a placebo-controlled blinded comparison with tiotropium. Respir Res 2010;11:135. [PubMed]
- Rossi A, Centanni S, Cerveri I, et al. Acute effects of indacaterol on lung hyperinflation in moderate COPD: a comparison with tiotropium. Respir Med 2012;106:84-90. [PubMed]
- O’Donnell DE, Casaburi R, Vincken W, et al. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir Med 2011;105:1030-6. [PubMed]
- Hataji O, Naito M, Ito K, et al. Indacaterol improves daily physical activity in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2013;8:1-5. [PubMed]
- Cazzola M, Rogliani P, Ruggeri P, et al. Chronic treatment with indacaterol and airway response to salbutamol in stable COPD. Respir Med 2013;107:848-53. [PubMed]
- Dahl R, Chung KF, Buhl R, et al. Efficacy of a new once-daily long-acting inhaled β2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax 2010;65:473-9. [PubMed]
- Donohue JF, Fogarty C, Lötvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010;182:155-62. [PubMed]
- Feldman G, Siler T, Prasad N, et al. Efficacy and safety of indacaterol 150 μg once-daily in COPD: a double-blind, randomised, 12-week study. BMC Pulm Med 2010;10:11. [PubMed]
- Buhl R, Dunn LJ, Disdier C, et al. Blinded 12-week comparison of once-daily indacaterol and tiotropium in COPD. Eur Respir J 2011;38:797-803. [PubMed]
- Chapman KR, Rennard SI, Dogra A, et al. Long-term safety and efficacy of indacaterol, a novel long-acting β2-agonist, in subjects with COPD: a randomized, placebo-controlled study. Chest 2011;140:68-75. [PubMed]
- Korn S, Kerwin E, Atis S, et al. Indacaterol once-daily provides superior efficacy to salmeterol twice-daily in COPD: a 12-week study. Respir Med 2011;105:719-26. [PubMed]
- Kornmann O, Dahl R, Centanni S, et al. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J 2011;37:273-9. [PubMed]
- Laforce C, Aumann J, Parreño LD, et al. Sustained 24-hour efficacy of once daily indacaterol (300 μg) in patients with chronic obstructive pulmonary disease: a randomized, crossover study. Pulm Pharmacol Ther 2011;24:162-8. [PubMed]
- Moen MD. Indacaterol: in chronic obstructive pulmonary disease. Drugs 2010;70:2269-80. [PubMed]
- Cope S, Capkun-Niggli G, Gale R, et al. Efficacy of once-daily indacaterol relative to alternative bronchodilators in COPD: a patient-level mixed treatment comparison. Value Health 2012;15:524-33. [PubMed]
- Han J, Dai L, Zhong N. Indacaterol on dyspnea in chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized placebo-controlled trials. BMC Pulm Med 2013;13:26. [PubMed]
- Mahler DA, Buhl R, Lawrence D, et al. Efficacy and safety of indacaterol and tiotropium in COPD patients according to dyspnoea severity. Pulm Pharmacol Ther 2013;26:348-55. [PubMed]
- Gotfried MH, Kerwin EM, Lawrence D, et al. Efficacy of indacaterol 75 μg once-daily on dyspnea and health status: results of two double-blind, placebo-controlled 12-week studies. COPD 2012;9:629-36. [PubMed]
- Worth H, Chung KF, Felser JM, et al. Cardio- and cerebrovascular safety of indacaterol vs formoterol, salmeterol, tiotropium and placebo in COPD. Respir Med 2011;105:571-9. [PubMed]
- Decramer M, Dahl R, Kornmann O, et al. Effects of long-acting bronchodilators in COPD patients according to COPD severity and ICS use. Respir Med 2013;107:223-32. [PubMed]
- Decramer M, Rossi A, Lawrence D, et al. Indacaterol therapy in patients with COPD not receiving other maintenance treatment. Respir Med 2012;106:1706-14. [PubMed]
- Donohue JF, Singh D, Kornmann O, et al. Safety of indacaterol in the treatment of patients with COPD. Int J Chron Obstruct Pulmon Dis 2011;6:477-92. [PubMed]
- Simoens S. Cost-effectiveness of pharmacotherapy for COPD in ambulatory care: a review. Eval Clin Pract 2013. [Epub ahead of print].
- Rodrigo GJ, Neffen H. Comparison of indacaterol with tiotropium or twice-daily long-acting beta-agonists for stable COPD: a systematic review. Chest 2012;142:1104-10. [PubMed]
- Price D, Gray A, Gale R, et al. Cost-utility analysis of indacaterol in Germany: a once-daily maintenance bronchodilator for patients with COPD. Respir Med 2011;105:1635-47. [PubMed]
- Price D, Asukai Y, Ananthapavan J, et al. Bronchodilator for patients with COPD, using real world evidence on resource use. Appl Health Econ Health Policy 2013;11:259-74. [PubMed]
- Cazzola M, Segreti A, Rogliani P. Comparative effectiveness of drugs for chronic obstructive pulmonary disease. Drugs Today (Barc) 2012;48:785-94. [PubMed]
- Rennard SI, Anderson W, ZuWallack R, et al. Use of a long-acting inhaled β2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:1087-92. [PubMed]
- Dahl R, Greefhorst LA, Nowak D, et al. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:778-84. [PubMed]
- Donohue JF, van Noord JA, Bateman ED, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002;122:47-55. [PubMed]
- Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versussalmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011;364:1093-103. [PubMed]
- Vogelmeier C, Magnussen H, LaForce C, et al. Profiling the bronchodilator effects of the novel ultra-long-acting β2-agonist indacaterol against established treatments in chronic obstructive pulmonary disease. Ther Adv Respir Dis 2011;5:345-57. [PubMed]
- Cazzola M, Brusasco V, Centanni S, et al. Project PriMo: sharing principles and practices of bronchodilator therapy monitoring in COPD: a consensus initiative for optimizing therapeutic appropriateness among Italian specialists. Pulm Pharmacol Ther 2013;26:218-28. [PubMed]
- Mahler DA, D’Urzo A, Bateman ED, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double-blind comparison. Thorax 2012;67:781-8. [PubMed]
- Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J 2013. [Epub ahead of print]. [PubMed]
- Cope S, Capkun-Niggli G, Gale R, et al. Comparative efficacy of indacaterol 150 μg and 300 μg versus fixed-dose combinations of formoterol + budesonide or salmeterol + fluticasone for the treatment of chronic obstructive pulmonary disease--a network meta-analysis. Int J Chron Obstruct Pulmon Dis 2011;6:329-44. [PubMed]
- Vogelmeir C, Bateman E, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (illuminate): a randomised, double-blind, parallel group study. Lancet Respir Med 2013;1:51-60.
- Puhan MA, Bachmann LM, Kleijnen J, et al. Inhaled drugs to reduce exacerbations in patients with chronic obstructive pulmonary disease: a network meta-analysis. BMC Med 2009;7:2. [PubMed]
- Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med 2013;1:199-209.