A comparison of three approaches for the treatment of early-stage thymomas: robot-assisted thoracic surgery, video-assisted thoracic surgery, and median sternotomy
Introduction
Thymomas originate in the thymus tissue, mainly located in the anterior mediastinum, comprising 20–30% of mediastinal masses in adults (1). The growth is mainly local and might be associated with metastases to the pleura, pericardium, or diaphragm. Distant metastases are rare (2). Extended thymectomy has been used as the standard surgical treatment for thymomas, which involves en bloc resection of the thymus and anterior mediastinal fat tissue. Thymectomy can be achieved by median sternotomy or video-assisted thoracic surgery (VATS) (3,4). However, there have been controversies in regards to the feasibility of complete dissection and possibility for future tumor seeding with VATS (5). Over the last few decades, the da Vinci surgical system has been extensively used in the surgical field, including, but not limited to cardiac and general surgeries (6,7). However, the literature pertaining to extended thymectomy with this robot-assisted technology (RATS) is limited, and very few studies have compared the outcomes achieved with VATS or RATS to those achieved with traditional MS.
Here, we retrospectively reviewed patients with early-stage thymomas who had undergone MS, VATS, or RATS in our hospital, comparing perioperative parameters (e.g., operative time, blood loss, and pleural drainage duration) and patient prognosis during follow-up to explore the most appropriate approach for the treatment of early-stage thymomas.
Methods
Patients
In this retrospective study, we reviewed 123 patients with Masaoka stage I and II thymomas (39 stage I, 84 stage II) who had undergone extended thymectomy at the Shanghai Chest Hospital between February 2009 and August 2014. RATS was performed on 51 patients, VATS was performed on 35 patients, and MS was performed on 37 patients. The type of procedure each patient underwent was selected by the patient. Cardio-respiratory function tests were performed for all patients, indicating that all were eligible for surgery. In addition, there were no distant metastases in any patient. Patients who underwent palliative surgery or did not undergo resection of the fat tissue were not included.
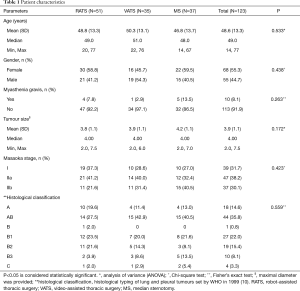
The demographic data, the presence of MG, tumor size, tumor staging, surgical approach, and adjuvant treatment were recorded. Tumor staging was set according to the Masaoka staging system (8); only patients with stages I and II tumors were included. In addition, the RATS, VATS, and MS groups were compared in terms of operative time, intra-operative blood loss volume, intra-operative complications, post-operative pleural drainage duration, post-operative pleural drainage volume, duration of hospital stay, and post-operative complications. Moreover, docking time (time period required for surgical cart placement and robotic arm positioning), and mortality were recorded.
Follow-up was made every 3 months for the first 6 months using chest CT (a total of twice). Afterwards, chest CT was performed every 6 months for a year (a total of twice), and then followed by the frequency of a CT once a year. The study was approved by Shanghai Chest Hospital Affiliated Shanghai Jiao tong University ethics committee [No. KS(P)1708].
Surgical technique
For all three groups, patients underwent an extended thymectomy, as described by Masaoka (9), under general anesthesia with double lumen intubation; an en bloc resection of the anterior mediastinal fat tissue, including the thymus, was performed. Dissection was performed bluntly from the pericardium and pleura. The adipose tissues around the upper poles of the thymus, around both brachiocephalic veins, and on the pericardium, were resected meticulously. If necessary, the pleural cavity was entered. The borders of resection were the diaphragm, caudally, the thyroid gland, cranially, and the phrenic nerves, laterally.
For MS (the traditional approach), the patient was placed in a supine position. After cutting the skin and subcutaneous tissue, the sternum was split.
For VATS, the patient was propped at 30–45 degrees in a semi-supine position. Generally, a right-sided approach was adopted. In the case of a left-sided thymoma, a left-sided approach can be adopted. A 3-port approach was used, with one observation port and two main operation ports. An observation port of 0.5–1 cm was made typically in the 6th intercostal space, at the anterior axillary line. This port was primarily used for placing the trocar lens of the thoracoscope into the thoracic cavity, and reserved for the placement of the upper chest tube. Two main operation ports were placed in the 5th intercostal space, at the midclavicular line, and in the 3rd intercostal space, at the anterior axillary line. An ultra-sonic dissector was used for resection.
For RATS, the Da Vinci® Surgical System was used to perform the extended thymectomy, which consists of several key components, including the following: an ergonomically designed console where the surgeon sits while operating, a patient-side cart where the patient lays during surgery, four interactive robotic arms, a high-definition 3D vision system, and proprietary EndoWrist® instruments. Da Vinci is powered by state-of-the-art robotic technology that allows the surgeon’s hand movements to be scaled, filtered, and translated into precise movements of the EndoWrist instruments working inside the patient’s body (Table 1).
Full table
The robot was docked first. The incision sites were the same as those used for VATS. The robotic endoscope was inserted in the 6th intercostal space, at an anterior axillary line for observation, and two robotic instrument arms were placed at the midclavicular line in the 5th intercostal space and at an anterior axillary line in the 3rd intercostal space. Cadiere forceps (EndoWrist®; Intuitive Surgical, USA) and a harmonic scalpel (HARMONIC® Ultra-Sonic Curved Shears and Blades; Ethicon Endo-Surgery, Inc., USA) were attached to the two instrument arms.
For VATS and RATS, the specimen was removed safely in a plastic retrieval bag, and then pulled out of the thoracic cavity. The surgical area was flushed with sterile distilled water repeatedly. For all three approaches, one chest tube (F32) was placed at the end of the operation. For patients with MG, treatment with pyridostigmine bromide and hormones was required to control symptoms. We measured operative time, intra-operative blood loss, post-operative pleura drainage duration and volume, and the length of the hospital stay; all the measurements were compared statistically.
Statistical analysis
Statistical analysis was performed using SAS version 9.2. For continuous variables, if a normal distribution and homogeneity of variance were assumed, analysis of variance (ANOVA) was performed. If not, a Kruskal Wallis test was performed for comparisons among the three groups. For comparisons between any two groups, the LSD test (homogeneity of variance) and Wilcoxon rank-sum test was performed. For categorical variables, Fisher’s exact test was used. P<0.05 was considered statistically significant. Comparisons were made for the three approaches in terms of patient characteristics and a series of intra- and post-operative parameters.
Results
Patient characteristics
In the present patient cohort, 51 patients underwent RATS, 35 underwent VATS, and 37 underwent MS. The mean age was 48.76 years old (range, 20–77 years) for RATS, 50.29 years old (range, 22–76 years) for VATS, and 46.76 years old (range, 14–67 years) for MS. There were no statistically significant differences among the three groups as far as patient characteristics, including tumor stage distribution, mean age, gender distribution, presence of MG, mean tumor size, and histological classification. No patients received adjuvant radiotherapy in any of the three groups. This detailed information is summarized in Table 1.
Comparisons of intra- and post-operative parameters
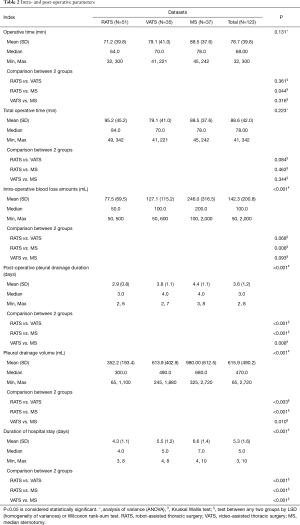
There were no deaths in any of the three groups, and no conversion to MS occurred in the RATS or VATS groups. The difference in mean operative time was not significantly different between the RATS, VATS, and MS groups. Under further analysis, operative time (not including the robot set-up time) was shorter for RATS compared to MS (71.2 vs. 88.5, P=0.044; not including the set-up time of RATS). All other parameters showed significant differences, which included intra-operative blood loss volume (77.5, 127.14, and 246.0 mL for RATS, VATS, and MS respectively, P<0.001), mean post-operative pleural drainage duration (2.9, 3.8, and 4.4 days, respectively, P<0.001), pleural drainage volume (352.2, 613.9, and 980.0 mL, respectively, P<0.001) and mean duration of hospital stay (4.3, 5.5, and 6.6 days, respectively, P<0.001) (Table 2). For parameters with significant differences among the three groups, some significance was also demonstrated when comparing two groups for parameters, such as operation time (RATS vs. MS, P=0.044), intra-operative blood loss volume (RATS vs. MS, P=0.008), post-operative pleural drainage duration (RATS vs. VATS, P<0.003; RATS vs. MS, P<0.001; VATS vs. MS, P=0.010), pleural drainage volume (RATS vs. VATS, P<0.001; RATS vs. MS, P<0.001; VATS vs. MS, P=0.008) and duration of hospital stay (RATS vs. VATS, P<0.001; RATS vs. MS, P<0.001; VATS vs. MS, P<0.001). According to these results, we can see that these parameters indicated that the outcomes of RATS were more favorable compared to VATS and MS, while outcomes for VATS were favorable to MS.
Full table
The percentages of patients with intra-operative complications were 3.9%, 5.4%, and 5.7% for RATS, MS, and VATS, respectively, including hemorrhage due to pleural adhesions in two patients in the RATS group, hemorrhage during operation in two patients in the MS and one patient in VATS group. Three patients (8.1%) had post-operative complications in the MS group, one with arrhythmia (atrial fibrillation), one with post-operative bleeding, and one with a pulmonary infection. The patient with arrhythmia returned to normal with amiodarone treatment. We provided nutrition support to the patient with bleeding, and the pleura drainage became normal in the second day. No blood transfusion was performed. The patient with pneumonia was cured after 1 week of antibiotic treatment. No post-operative complications occurred in the VATS & RATS group. No statistically significant differences occurred between groups in terms of intra-operative complications as determined by Fisher’s exact tests (P=1.00, P>0.05). All complications were Grade 2 in the Clavien-Dindo system.
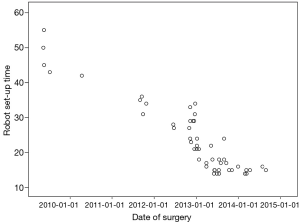
For the RATS group, the operative and the robot set-up time curve was presented (Figure 1). The abscissa axis represents 51 patients in the RATS group in chronological order. The longitudinal axis represents operative and docking time. As the number of patients increased, both robot set-up time and operative time declined. An operative time of 300 minutes occurred in a case with hemorrhage due to pleural adhesions. The mean follow-up periods for RATS, VATS and MS were 420.9±469.1, 701.3±381.95, and 818.3±592.5 days, respectively. During these periods, all patients survived without recurrence.
Discussion
Thymoma is a rare epithelia neoplasm of the thymus gland, and it is the most common tumor of the anterior superior mediastinum, comprising 20–30% of mediastinal masses in adults (1). With the advantages of minimal invasiveness and rapid recovery, VATS has been adopted more often in recent years to treat early-stage thymomas. In the early 1990s, Roviaro (11) reported on video-assisted thymectomy. Comparing VATS and MS for the treatment of MG, it has been shown that VATS is typically associated with reduced blood loss and a shorter operative time and hospital stay, while VATS may also lower the incidence rate of post-operative complications by reducing tissue damage, post-operative pain, and the risk of infection (12,13). When comparing RATS (the newest MG surgical therapy) and MS for the treatment of MG, it has been demonstrated that RATS has favorable outcomes in terms of surgical complications and hospital stay (14).
To date, most published studies have compared between MS and VATS or between MS and RATS without comparing among the three surgical techniques. In addition, MS is typically recommended for early-stage thymomas, often instead of RATS. To our knowledge, this is the first study to compare these three approaches for the treatment of thymomas. Moreover, for the treatment of early-stage, especially stage II thymomas, robotic-assisted surgeries are performed much less frequently in Mainland China compared to Western countries. Our hospital performed the first robot-assisted thoracic surgery in Mainland China. The differences between three techniques were summarized in Table 3.

Full table
According to the results of this study, intra-operative blood loss volume, mean post-operative pleural drainage duration, pleural drainage volume, and the mean duration of hospital stay show more favorable results in the RATS group versus the VATS or MS group (all with P<0.05). Furthermore, outcomes were more favorable in the VATS compared to the MS group (all with P<0.05). These results are consistent with other studies. A recent comparative study (15) of robot-assisted thymectomy versus trans-sternal thymectomy in patients undergoing extended thymectomy demonstrated that intra-operative blood loss was significantly higher in the trans-sternal group (151.4 vs. 41.7 mL, P=0.01). There were 20 post-operative complications and 1 post-operative death in the trans-sternal group and 1 post-operative complication in the robot-assisted group (P=0.001). The duration of hospital stay was 4 days (range, 2–27 days) in the trans-sternal group and 1 day (range, 1–7 days) in the robot-assisted group (P=0.002). In our study, intra-operative blood loss and duration of hospital stay showed more favorable outcomes in the RATS compared to the MS group (77.5 vs. 246.0 mL, P<0.001 and 4.3 vs. 6.6 days, P<0.001, respectively). Moreover, three patients (8.1%) had post-operative complications in the MS group compared to none (0.0%) in the RATS group. Another recent comparative study (16) of VATS versus RATS during the surgical treatment of Masaoka stage I thymomas showed that the post-operative hospital stay was significantly shorter in the RATS group (3.7 vs. 6.7 days; P<0.01), and the post-operative pleural drainage volume in the RATS group was significantly less than in the VATS group. In our study, the duration of hospital stay and post-operative pleural drainage volume were also more favorable in the RATS versus the VATS group (4.3 vs. 5.5 days respectively, P<0.001; 352.2 vs. 613.9 mL respectively, P<0.003). The reason why post-operative pleura drainage was reduced in the RATS group may be due to the enhanced 3D vision; the console surgeon can see the detail more clearly than with 2D VATS. Furthermore, the RATS incisions are smaller and less invasive than VATS. In a single arm retrospective study of 14 patients undergoing robotic thymectomy for the treatment of early-stage thymomas (17), the total operative time was 139 min, which is longer than the average of 95.2 min in our study; no intra-operative complications or death occurred in this study, compared to 1 patient (4.3%) with the complication of hemorrhage due to pleural adhesions in our study. Minor complications occurred in two patients (14.2%) in this study due to pleural effusion compared to no post-operative complications in the RATS group from our study. The mean hospital stay in this study was 4.0 days compared to 4.3 days in our study. As for the comparisons between the VATS group and MS group, our data were also consistent with published studies (18-20).
In terms of operative time, the total time (95.2 min) in the RATS group was greater than the MS (88.5 min) and VATS (79.1 min) groups, although this was not statistically significant (P=0.223). However, there was a trend towards decreased set-up time and operative time as the number of patients increased. Since robotic surgery is a new approach, it requires time for gaining skills and experience. In addition, one patient in the RATS group had excessive bleeding during the operation due to a pre-existing pleural adhesion, resulting in a much longer operative time (300 min). This value increased the average operative time for the RATS group. Ultimately, patients in the RATS group recovered faster under a minimally invasive procedure in terms of intra- and post-operative parameters, which was more favorable than the outcomes in the VATS and MS groups.
The controversies surrounding minimally invasive surgeries mainly focus on the oncological concerns (possible breach of tumor capsule, risk of tumor seeding) (21). It has been reported that for the treatment of stage I and II thymomas, there were no significant differences in the 5-year survival, 5-year recurrence-free survival (19), or mean survival time (4) between the VATS group and open surgery group. In addition, robots have more technical advantages with three-dimensional vision systems and multi-articulated instruments, which allows an open-like intervention, while robotic extended thymectomy has been shown to be feasible and safe (22,23). In a recent comparative study, no early recurrences were observed during the postoperative follow-up period of 17.5 months (range, 6–48 months) in the RATS group and 25.2 months (range, 6–48 months) in the VATS group (16). In our study, during the follow-up period (420.9±469.1, 701.3±556.9, and 818.3±592.5 days for RATS, RATS and MS, respectively), all patients survived without any recurrences. Hence, the application of RATS may be promising for the future treatment of stage I and II thymomas. However, due to the unpredictable nature of thymomas, a longer follow-up period is required to evaluate oncological outcomes. The present study also has other limitations, such as the retrospective, non-randomized design, and relatively small sample size. Further studies will be needed with a longer duration of follow-up visits and an expanded sample size.
Conclusions
To our knowledge, this is one of a small number of studies comparing three surgical approaches (RATS, MS, VATS) for the treatment of early stage thymomas. RATS and VATS appeared feasible and safe for the resection of such tumors. A series of intra- and post-operative parameters showed more favorable outcomes for RATS compared to VATS and VATS compared to MS. Oncological outcomes appeared equal among the three groups, but a longer follow-up period is required.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Shanghai Chest Hospital Affiliated Shanghai Jiao tong University ethics committee [No. KS(P)1708].
References
- Levine GD, Rosai J. Thymic hyperplasia and neoplasia: a review of current concepts. Hum Pathol 1978;9:495-515. [Crossref] [PubMed]
- Paerson FG, Cooper JD, Deslauries J, et al. Thoracic Surgery. 2nd ed. Philadelphia: Churchill Livingstone, 2002.
- Yim AP. Video-assisted thoracoscopic resection of anterior mediastinal masses. Int Surg 1996;81:350-3. [PubMed]
- Cheng YJ, Kao EL, Chou SH. Videothoracoscopic resection of stage II thymoma: prospective comparison of the results between thoracoscopy and open methods. Chest 2005;128:3010-2. [Crossref] [PubMed]
- Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg 2008;86:673-84. [Crossref] [PubMed]
- Boyd WD, Kodera K, Stahl KD, et al. Current status and future directions in computer-enhanced video- and robotic-assisted coronary bypass surgery. Semin Thorac Cardiovasc Surg 2002;14:101-9. [Crossref] [PubMed]
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Masaoka A, Yamakawa Y, Niwa H, et al. Extended thymectomy for myasthenia gravis patients: a 20-year review. Ann Thorac Surg 1996;62:853-9. [Crossref] [PubMed]
- Travis WD, Colby TV, Corrin B. Histological Typing of Lung and Pleural Tumours. 3rd ed. World Health Organization International Histological Classification of Tumours. Springer-Verlag, 1999.
- Roviaro GC, Varoli F, Vergani C, et al. Thoracoscopic thymectomy. Semin Laparosc Surg 1997;4:219-22.
- Lin MW, Chang YL, Huang PM, et al. Thymectomy for non-thymomatous myasthenia gravis: a comparison of surgical methods and analysis of prognostic factors. Eur J Cardiothorac Surg 2010;37:7-12. [Crossref] [PubMed]
- Zahid I, Sharif S, Routledge T, et al. Video-assisted thoracoscopic surgery or transsternal thymectomy in the treatment of myasthenia gravis? Interact Cardiovasc Thorac Surg 2011;12:40-6. [Crossref] [PubMed]
- Cakar F, Werner P, Augustin F, et al. A comparison of outcomes after robotic open extended thymectomy for myasthenia gravis. Eur J Cardiothorac Surg 2007;31:501-4; discussion 504-5. [Crossref] [PubMed]
- Weksler B, Tavares J, Newhook TE, et al. Robot-assisted thymectomy is superior to transsternal thymectomy. Surg Endosc 2012;26:261-6. [Crossref] [PubMed]
- Ye B, Tantai JC, Li W, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J Surg Oncol 2013;11:157. [Crossref] [PubMed]
- Mussi A, Fanucchi O, Davini F, et al. Robotic extended thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2012;41:e43-6; discussion e47.
- Agasthian T, Lin SJ. Clinical outcome of video-assisted thymectomy for myasthenia gravis and thymoma. Asian Cardiovasc Thorac Ann 2010;18:234-9. [Crossref] [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [Crossref] [PubMed]
- Iablonskiĭ PK, Pishchik VG, Nuraliev SM. Comparative assessment of the effectiveness of traditional and videothoracoscopic thymectomies in complex treatment of myasthenic thymomas. Vestn Khir Im I I Grek 2005;164:38-42. [PubMed]
- Roviaro G, Varoli F, Nucca O, et al. Videothoracoscopic approach to primary mediastinal pathology. Chest 2000;117:1179-83. [Crossref] [PubMed]
- Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-65; discussion 265-6. [Crossref] [PubMed]
- Rea F, Marulli G, Bortolotti L, et al. Experience with the "da Vinci" robotic system for thymectomy in patients with myasthenia gravis: report of 33 cases. Ann Thorac Surg 2006;81:455-9. [Crossref] [PubMed]


