Non responders to neoadjuvant chemoradiation for esophageal cancer: why better prediction is necessary
Introduction
Esophageal cancer (EC) is currently ranked as the seventh cancer-related cause of death worldwide. The life expectancy for patients with clinically resectable, locally advanced EC is still dismal with a 5-year overall survival (OS) rate of only up to 40% (1-3). For those patients neoadjuvant chemoradiation (nCRT) combined with surgery is considered as the optimal curative intended treatment according to the regimen used in the Dutch randomized controlled trial “Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study” (CROSS) (4,5).
Unfortunately, only 40–60% of all patients respond to nCRT and approximately 25–33% of all patients achieve a pathologic complete response (pCR) (6). Pathological response rate is usually classified according to the Mandard classification, which defines pCR as tumor regression grade (TRG) 1 in absence of malignant cells in the resection specimen (7). Several studies have shown that pCR is independently associated with survival, both the 5-year OS and disease free survival (6,8,9). In literature, squamous cell carcinoma (SCC) and female gender are identified as predictive factors for pCR (8,10-14), there is however limited knowledge on factors that can be used to predict which patients will achieve a pathologic non response (pNR). The effect of nCRT in patients with a pNR remains unclear. Tumors without response to nCRT may imply insensitivity for radiotherapy and likeliness of high metastatic potential, developing local regrowth or distance metastases in the nCRT period (15). Better selection of patients for nCRT is a strong focus in current EC research, aiming to avoid morbidity and mortality from nCRT in patients who will not benefit from this treatment.
Therefore, the primary aim of this study is to compare oncological outcomes of EC patients with pNR to nCRT with patients who underwent primary esophagectomy (without nCRT) to evaluate the potential benefit of nCRT in patients with a pNR. Furthermore, the secondary aim is to identify predictive clinicopathological factors for pNR and pCR to nCRT for a better patient selection.
Methods
Primary study population
After Institutional Review Board approval, all files of patients who underwent nCRT followed by resection for EC at the VU University medical center (VUmc) in Amsterdam between January 2005 and March 2016 were retrospectively reviewed. The treatment regime consisted of radiotherapy with a total dose of 41.4 Gy given in 23 fractions of 1.8 Gy 5 times per week; combined with a weekly course of Paclitaxel (50 mg/m2) and Carboplatin (area under the curve, 2) during the treatment period. Patients underwent a surgical resection with curative intent within 6–8 weeks after completing nCRT. For surgical resection either a minimally invasive or open transthoracic or transhiatal procedure was performed. In total 206 consecutive patients staged as cT1N+/T2-4a/N0-3 and M0 were included in this analysis. Patients who underwent nCRT without complementary esophagectomy were excluded in this study.
Matching historical cohort
In a separate analysis included in this study, the results of patients with a pNR after nCRT were compared with a propensity score matched historical cohort of 218 primary surgically treated patients from the University Medical Center Groningen (UMCG), in the Netherlands. In the primary esophagectomy group, patients were treated through a transthoracic esophagectomy with two-field lymphadenectomy without nCRT, because it was not yet standard treatment at that time (before 2006). Only patients staged cT1N+/T2-4a/N0-3 and M0 were included.
Staging procedure
Staging was performed according to the American Joint Commission on Cancer (AJCC) (7th edition). Pretreatment clinical staging in VUmc cohort consisted of endoscopic ultrasonography (EUS), computed tomography (CT) scans and/or positron emission tomography (PET)/CT scans.
Histopathological examination
The pathological reports of patients provided by the pathologist were reviewed. TRG was classified according to the Mandard classification (7). The response scale ranges from Mandard 1 for a complete response to Mandard 5 for non-response. In this study, Mandard 1 was scored as a pCR, Mandard 2 and 3 were scored as a non-pCR and Mandard 4 and 5 were scored as limited or no response (the abbreviation pNR will be used in this study). Furthermore, other parameters including T- and N-stage, radicality of the resection (R) and the number of positive lymph nodes were examined.
Follow up
All patients were monitored periodically according to the Dutch national guidelines for follow up. In the first year after treatment, patients were seen every 3 months. Patients were seen every 6 months in the second year. And up to 5 years after treatment, patients were seen yearly. Follow up time was calculated from the date of the start of nCRT up to the date of all-cause death or to the last day of follow up at the outpatient clinic.
Statistics
Statistical analyses were performed using PASW Statistics software (version 22.0; SPSS, Inc., Chicago, IL, USA). Survival curves were estimated according to the Kaplan-Meier method and subsequently compared using the log-rank test. Matched cohorts were created according to the propensity score, which is a balancing score implemented in the Statistics software pack (16,17). An one-to-one matched group of 68 patients was formed from the 218 patients in the primary surgically treated group based on sex, age, histological type, cT- and cN-stage. Four patients were excluded in this analysis due to missing data in the factors were the propensity scored matching was based on. The OS rate was calculated from the date of the start of treatment (i.e., start of nCRT in VUmc cohort and day of surgery in UMCG cohort) up to the date of all-cause death or to the last day of follow up at the outpatient clinic. A multivariate regression model was used to examine associations between clinicopathological parameters and pNR or pCR. All statistical tests were conducted two-sided, and a P value <0.05 was considered significant.
Results
Patient and tumor characteristics
The characteristics of the 206 patients are presented in Table 1. This study included 157 male patients (76.2%) and 49 female patients (23.8%). The median age at diagnosis was 63 years (range 37.1–83.2 years). In total, 138 patients had an adenocarcinoma (67%) and 66 patients a SCC (32%). In 72 (35.1%) cases a pCR and in 72 cases (35.1%) a pNR was found. Thirty-four patients with a pCR had a SCC.
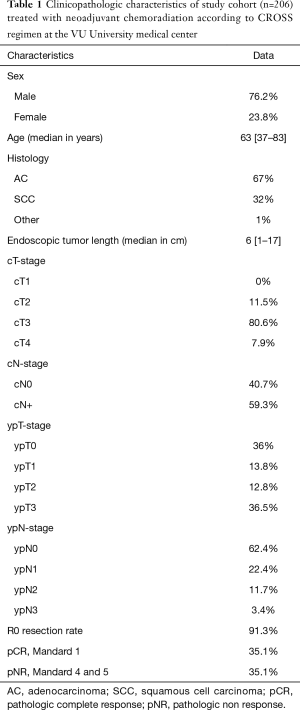
Full table
OS outcomes
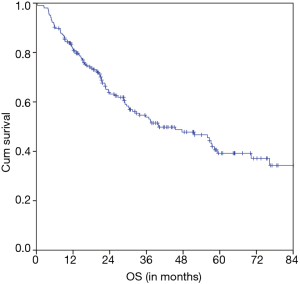
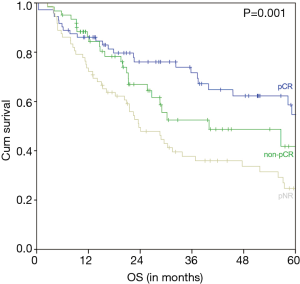
The estimated 5-year OS of all 206 nCRT patients was 39.2% and the median OS was 40.0 months (95% CI 22.7–57.2 months) (Figure 1). A significant difference of the estimated 5-year OS of 54.7% (in patients with a pCR) versus 41.8% (in patients with a non-pCR) versus 24.7% (in patients with a pNR) was observed (P=0.001) (Figure 2).
OS in patients with a pNR versus primary esophagectomy patients
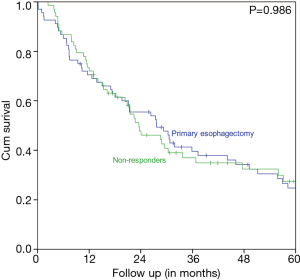
Characteristics of the pNR VUmc group and the matched primary esophagectomy UMCG cohort are displayed in Table 2. The two matched groups are statistically comparable. Figure 3 shows that patients with pNR did not have a better survival rate than the matched primary esophagectomy patients. In the pNR group the estimated 5-year OS rate was 27.4% versus 24.7% in the primary esophagectomy group. The median survival was 23.6 months (95% CI 16.3–30.8 months) in the pNR group versus 27.7 months (95% CI 17.5–37.8 months) in the primary esophagectomy group. This difference was not statistically significant (P=0.986).
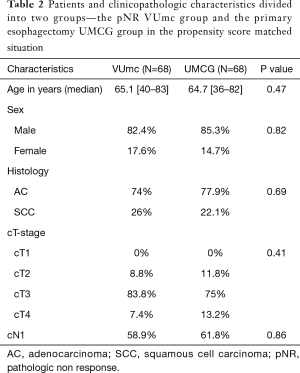
Full table
Prediction of pNR and pCR
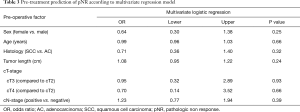
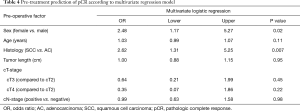
Multivariate analysis was performed to determine predictive factors for pNR (Mandard 4 and 5). Included factors were gender, age, histological tumor type, tumor length, cT and cN-stage. The multivariate regression model (Table 3) showed that there were no predictive factors in this cohort for pNR. Furthermore, multivariate analysis was performed to determine predictive factors for pCR (Mandard 1). Included factors were the same as the factors in the pNR model. Multivariate analysis (Table 4) identified female gender (OR 2.5, 95% CI 1.2–5.3) and SCC (OR 2.6, 95% CI 1.3–5.3) as independent predictive factors for pCR.
Full table
Full table
Discussion
In the present study, OS was correlated with the degree of pathologic response to nCRT. Patients with a pCR have a significantly improved survival compared with patients with pNR. This is consistent with data published from previous studies (6,8,9). In this study, 35.1% of patients achieved pCR which is comparable with the recent CROSS trial data (6). Of those patients, 47.2% had a SCC and the estimated 5-year OS rate of the whole group was 54.7%. With a median OS of 40.0 months in our group, we report a lower OS than was observed and described by Shapiro et al. in their update of the long-term results of the CROSS trial. However, no information is noted about the distribution of the TRG which could explain this difference (18).
Current guidelines for EC recommend nCRT for all patients with stage > T1b or with node-positive disease (19). While this approach may improve outcome for the majority of patients, as demonstrated in the long term outcome of the CROSS trial, it has not consistently benefitted all patients (18). In addition to the current literature, the results of this study confirm that there is no survival advantage after nCRT for patients with pNR. An important limitation of the present study is the retrospective character which could introduce selection bias. A recent study of Dittrick et al. compared unmatched non-responders to primary esophagectomy patients and found no benefit of nCRT for non-responding patients (20). In contrast to that study, the results of this study are achieved by performing propensity-matched analysis between both groups and therefore more reliable.
An important question for the subgroup of complete nCRT responders is whether there is room for a “wait and see” strategy with close monitoring during follow up and salvage surgery if necessary (21,22). A study of Chao et al. showed that 42% of patients with a near pCR (defined as residual cancer representing less than 10 per cent of the original tumor area) did have a disease in the layers of the esophageal wall besides the mucosal layer. This emphasizes the importance to distinguish between tumor and fibrosis in the deeper layers. Unfortunately, at this moment, imaging technics are not accurate enough to show the difference between a pCR and a near pCR. Taking biopsies have the risk of bleeding, besides the fact that there is a high probability of a sampling error (23).
If patients are non-responders, they were unnecessarily exposed to the nCRT related side effects, without having the oncological benefits. It is therefore important to select patients before treatment is given and one of the aims of this study was therefore to identify factors which can predict a pNR. No pre-treatment or patient specific factors predicting pNR were identified in this cohort using the multivariate regression model. To detect and select these patients in advance, a different treatment approach can be used, e.g., they may proceed to surgery directly potentially followed by adjuvant radiation therapy (24). In addition, a recent study by Goodman et al. showed that early response assessment by using PET imaging 6 weeks after start of nCRT improved identifying more effective new regimens for EC. Those who did not respond to the assigned therapy were switched to the alternative regimen for nCRT and by switching more patients achieved a pCR. By using this approach, more patients can be treated with a personalized and effective treatment regimen (25).
In concordance with the literature, this data demonstrate that female gender and SCCs are the strongest pre-treatment predictive factors for pCR (10,14,26-28). However the number of females with SCC is too small in this cohort to confirm this with a significant better OS outcome.
In conclusion, in this cohort the OS of patients with a pNR to nCRT is comparable with patients who only had a primary esophagectomy. Female patients and patients with a SCC benefit most from nCRT. Although nCRT does not negatively impact the OS of a patient with a pNR, the patient still has an increased morbidity as a result of nCRT. Therefore, it is essential to identify factors that predict pNR to nCRT. The ability to predict pNR will enable tailored and personalized care preventing unnecessary nCRT with increased morbidity and equally nCRT will be applied to patients that do benefit from nCRT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The medical ethics committee of the VU University medical center approved the protocol in 2013 (registration number 2013.076).
References
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol 2007;25:3719-25. [Crossref] [PubMed]
- Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062-7. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Vallböhmer D, Hölscher AH, DeMeester S, et al. A multicenter study of survival after neoadjuvant radiotherapy/chemotherapy and esophagectomy for ypT0N0M0R0 esophageal cancer. Ann Surg 2010;252:744-9. [Crossref] [PubMed]
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [Crossref] [PubMed]
- Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol 2005;23:4330-7. [Crossref] [PubMed]
- Geh JI, Crellin AM, Glynne-Jones R. Preoperative (neoadjuvant) chemoradiotherapy in oesophageal cancer. Br J Surg 2001;88:338-56. [Crossref] [PubMed]
- Rohatgi P, Swisher SG, Correa AM, et al. Characterization of pathologic complete response after preoperative chemoradiotherapy in carcinoma of the esophagus and outcome after pathologic complete response. Cancer 2005;104:2365-72. [Crossref] [PubMed]
- Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005;103:1347-55. [Crossref] [PubMed]
- Rizk NP, Venkatraman E, Bains MS, et al. American Joint Committee on Cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol 2007;25:507-12. [Crossref] [PubMed]
- Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg 2009;87:392-8; discussion 398-9. [Crossref] [PubMed]
- Ajani JA, Correa AM, Hofstetter WL, et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol 2012;23:2638-42. [Crossref] [PubMed]
- Rohatgi PR, Swisher SG, Correa AM, et al. Failure patterns correlate with the proportion of residual carcinoma after preoperative chemoradiotherapy for carcinoma of the esophagus. Cancer 2005;104:1349-55. [Crossref] [PubMed]
- D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-81. [Crossref] [PubMed]
- Weitzen S, Lapane KL, Toledano AY, et al. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf 2004;13:841-53. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [Crossref] [PubMed]
- Dittrick GW, Weber JM, Shridhar R, et al. Pathologic nonresponders after neoadjuvant chemoradiation for esophageal cancer demonstrate no survival benefit compared with patients treated with primary esophagectomy. Ann Surg Oncol 2012;19:1678-84. [Crossref] [PubMed]
- Castoro C, Scarpa M, Cagol M, et al. Complete clinical response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic oesophagus: is surgery always necessary? J Gastrointest Surg 2013;17:1375-81. [Crossref] [PubMed]
- Noordman BJ, Shapiro J, Spaander MC, et al. Accuracy of Detecting Residual Disease After Cross Neoadjuvant Chemoradiotherapy for Esophageal Cancer (preSANO Trial): Rationale and Protocol. JMIR Res Protoc 2015;4:e79. [Crossref] [PubMed]
- Chao YK, Chang Y, Yeh CJ, et al. Characterization of residual tumours at the primary site in patients with a near pathological complete response after neoadjuvant chemoradiotherapy for oesophageal cancer. Br J Surg 2016;103:1874-9. [Crossref] [PubMed]
- Shridhar R, Weber J, Hoffe SE, et al. Adjuvant radiation therapy and lymphadenectomy in esophageal cancer: a SEER database analysis. J Gastrointest Surg 2013;17:1339-45. [Crossref] [PubMed]
- Goodman KA, Niedzwiecki D, Hall N, et al. Initial results of CALGB 80803 (Alliance): A randomized phase II trial of PET scan-directed combined modality therapy for esophageal cancer. J Clin Oncol 2017;suppl 4S:abstract 1.
- Kesler KA, Helft PR, Werner EA, et al. A retrospective analysis of locally advanced esophageal cancer patients treated with neoadjuvant chemoradiation therapy followed by surgery or surgery alone. Ann Thorac Surg 2005;79:1116-21. [Crossref] [PubMed]
- Rohatgi PR, Swisher SG, Correa AM, et al. Histologic subtypes as determinants of outcome in esophageal carcinoma patients with pathologic complete response after preoperative chemoradiotherapy. Cancer 2006;106:552-8. [Crossref] [PubMed]
- Eil R, Diggs BS, Wang SJ, et al. Nomogram for predicting the benefit of neoadjuvant chemoradiotherapy for patients with esophageal cancer: a SEER-Medicare analysis. Cancer 2014;120:492-8. [Crossref] [PubMed]


