Surgical anatomy of the omental bursa and the stomach based on a minimally invasive approach: different approaches and technical steps to resection and lymphadenectomy
Introduction
During minimally invasive surgery (MIS) of the upper gastrointestinal (GI) tract, such as esophagectomy, gastrectomy, pancreatectomy, and transverse colectomy, it is imperative to have a thorough knowledge of the omental bursa (or: lesser sac) in order to perform an adequate dissection of those organs and an appropriate lymphadenectomy. Yet the surgical anatomy of the omental bursa is very complex as the rotational embryological development of the upper abdominal organs results in a crossroads of these organs with accompanying vessels and lymph nodes (1), hence making surgery around these organs quite difficult.
Our observation and dissection of the omental bursa during MIS prompted a descriptive study of this area, based on laparoscopic observation, with the aim to devise an understandable surgical anatomical concept. From our surgical-anatomic point of view we deemed the following three points important to know: (I) which of the various approaches can be used to enter the omental bursa surgically and to perform the most feasible and complete lymphadenectomy? (II) What are the surgical boundaries and anatomical landmarks of the omental bursa as seen during MIS? And (III) is the excision of the bursa (bursectomy) necessary for oncological reasons?
In this study, we aim to answer these three questions by video observations, a cadaver study, and a review of the current literature.
Methods
Observational study
One of the surgeons (Miguel A. Cuesta) involved in this study reviewed unedited videos of 20 consecutive patients undergoing MIS [2013–2014]: 10 esophagectomies (MIE), 5 laparoscopic gastrectomies (MIG, 4 total and 1 partial), and 5 undergoing distal pancreatectomy (MIDP). Subsequently, a prospective verification study was performed in 5 consecutive patients with gastric cancer and intervened by MIG [2016]. Institutional review board approval and informed consent were waived.
The MIEs were performed by laparoscopic and thoracoscopic approaches in prone position. During the esophageal resections, a D1+ lymphadenectomy of the celiac trunk had been performed followed by creation of a gastric conduit. All patients who underwent a MIE received neoadjuvant chemo-radiotherapy according to the CROSS schemeJeny (2). All patients who underwent MIG received perioperative chemotherapy according to the MAGIC schemeJeny (3) and a D2 lymphadenectomy (4).
The anatomical landmarks, peritoneal folds and vascular structures of the omental bursa as found during the prospective study and cadaver study were scored in all studied retrospective (20 patients) and prospective (5 patients) videos, including the; gastropancreatic fold (covering the celiac trunk), hepatic artery proper, right gastric artery, splenic artery and vein, right gastroomental fold with the right gastroepiploic (gastroomental) vessels, left gastroomental fold with the left gastroepiploic vessels, and short gastric arteries fold (with the short gastric vessels).
Lymphadenectomy, dissection and reconstruction
Lymph nodes were defined according to the Japanese gastric cancer guidelines (4,5), which offer us a complete set of guidelines not only concerning indication and procedures but also a detailed description of the location of lymph nodes and accordingly lymphadenectomy for total, and distal gastrectomy. After entering the bursa surgically, opening of the peritoneum above the pancreas permits dissection of the common hepatic artery nodes (station 8a) and from there dissection proceeds up to the liver along the right gastric artery up to the level of proper hepatic artery (station 12a). Proceeding from the common hepatic artery to the left, the celiac trunk is approached (station 9), the left gastric vein and artery are dissected free and then divided (station 7). The next step is to continue the lymphadenectomy along the splenic artery—as far as possible—on the superior edge of the body of the pancreas (station 11p and 11d) and to the splenic hilum (station 10). After dissection of the D2 lymph node stations, oncological dissection of the involved organ must be performed. In the case of gastric cancer, this includes resection of the stomach with all the D1 lymph nodes depending of the level of gastric resection.
The next step during MIG is to divide the proximal duodenum and the proximal stomach or the esophagus, depending of the type of gastric resection, in order to proceed with the anastomosis. In the case of MIE, the D1+ lymphadenectomy is followed by dissection of the greater curvature with preservation of the right gastroepiploic vessels and creation of a well vascularised gastric conduit.
Cadaver study
A cadaver’s abdomen was sliced in transverse cross sections of 1 centimeter thickness, aiming to verify the in vivo findings and verify the concept of the boundaries of the omental bursa.
Literature review
The PubMed database was searched for: “Omental Bursa” AND “Gastric Cancer” along with their synonyms or abbreviations. No additional search software or special features were used. One investigator (Hylke J. F. Brenkman) performed the screening and article selection procedures. All articles that fulfilled the eligibility criteria were included in the systematic review. The final search was performed on October 17, 2016.
Results
Patients
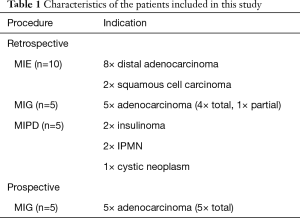
The patient characteristics of the retrospective and prospective study are depicted in Table 1. Ten patients had distally located adenocarcinoma of the esophagus; ten patients had gastric carcinoma and five patients had distal pancreatic tumors. In the retrospective study, lymphadenectomy of the celiac trunk was performed through the gastrocolic ligament in the first 7 patients (6 esophagectomies and 1 gastrectomy), whereas in remaining 13 patients lymphadenectomy was performed through the hepatogastric ligament.
Full table
Observational study
Anatomical findings of the retrospective and prospective score system are depicted in Table 2. Visualization of both the left gastroomental and short gastric folds during MIE was relative low (60%), just as the visualization of the left gastroomental fold during the gastrectomies (retrospective study) and distal pancreatectomy (40% and 60% respectively). Adequate dissection of lymph node station 6 was achieved completely in 9 of the 10 patients who underwent a gastrectomy, and lymphadenectomy of station 10 was not done in one patient during total gastrectomy. Concerning anatomical variations which were found during surgical dissection, 3 patients had an accessory left hepatic artery which originated from the left gastric artery, in 30% of the patients the splenic artery initially had a retropancreatic course and in 60% of the patients it showed a tortuous meander-shaped course along the superior edge of the body and tail of the pancreas up to the splenic hilum.

Full table
Surgical approach of the omental bursa
During minimally invasive upper GI surgery, the omental bursa can be approached in three ways:
- By opening the hepatogastric ligament (pars flaccida of the lesser omentum);
- Through the gastrocolic and gastrosplenic ligament, and;
- By opening the transverse mesocolon at the level of the pancreas.
The first option starts by opening the lesser omentum and retraction of the stomach to the left, so that the superior edge of the pancreas can be visualized. A necessary condition is an adequate retraction of the stomach. In the case of esophageal cancer, the hepatogastric ligament of the lesser omentum can be divided at the level of the gastric angular notch in order to create more space for retraction.
The second option starts by entering the omental bursa through the gastrocolic and/or gastrosplenic ligament. This permits a good approach of the celiac trunk with its three arterial branches, but to a lesser extent visualization of the lymph nodes along the hepatic artery to the hilum of the liver. In many cases a combination of the first and second approach is necessary.
The first two approaches are used in upper GI surgery, being the approach through the gastrosplenic ligament used to create any type of fundoplication during surgery for Gastroesophageal Reflux disease. The last option is used in colorectal surgery for mobilization of the splenic flexure.
Anatomical structures of the omental bursa as observed during MIS
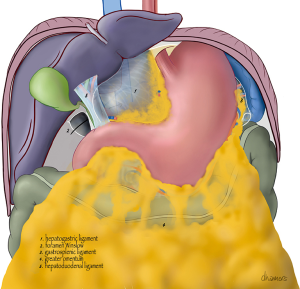
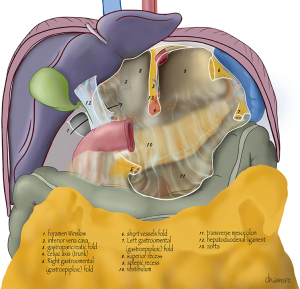
A schematic anterior view of the bursa is shown in Figures 1,2. If the omental bursa is visualized as a square box, the anterior boundary of the omental bursa consists of the hepatogastric ligament (pars flaccida), the posterior wall of the stomach (main part), the gastrocolic ligament with the gastroepiploic vessels, and the gastrosplenic ligament with the short gastric vessels.
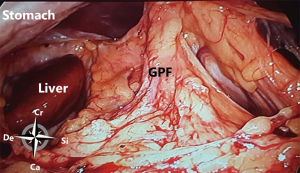
At the posterior wall, at the most cranial part, the gastropancreatic fold, from the aorta to the lesser gastric curvature, contains the celiac trunk and left gastric vessels and divides the cranial part of the omental bursa in two compartments (Figure 3). The right space is commonly named the superior recess, whereas the left space is commonly known as the splenic recess. The connection between the superior recess and the splenic recess is at the level of the pancreas just caudal to the celiac trunk; this part of the omental bursa is called the vestibulum. During MIS, one enters the superior recess by opening the hepatogastric ligament (pars flaccida), and the splenic recess by opening the gastrosplenic ligament.
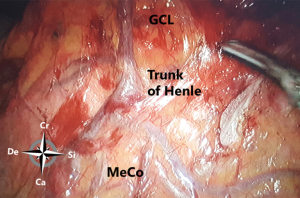
From caudal to cranial, the posterior wall and floor of the splenic recess consists of the transverse mesocolon up to the inferior edge of the pancreas, the splenorenal ligament with the splenic artery and vein, and more cranially the retroperitoneum (covering the left adrenal gland and left kidney) up to the diaphragm. The left lateral wall is formed in the upper part by the gastrosplenic ligament, the short gastric vessels fold and the left gastroomental fold (Figure 4). These two folds contain the short gastric vessels and the left gastroepiploic vessels, respectively originating from the distal part of the splenic artery and running upward and downward to the greater gastric curvature. At the level of the head of the pancreas the right gastroomental fold, containing the right gastroepiploic vessels (originating from the gastroduodenal artery once this artery has passed behind the duodenum) is located at the right inferior side of the omental bursa (Figure 4). This part of the omental bursa is named the inferior recess. During MIS, one enters the inferior recess by opening the gastrocolic ligament or by incision of the transverse mesocolon at the level of the pancreas (Figure 5).
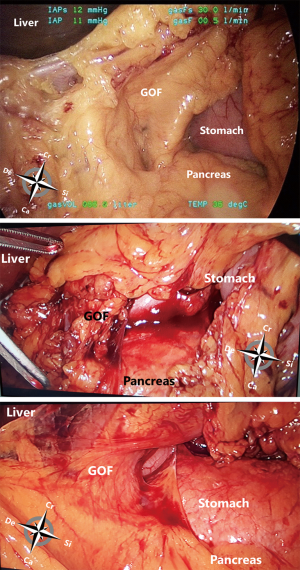
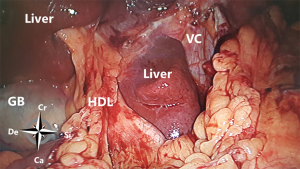
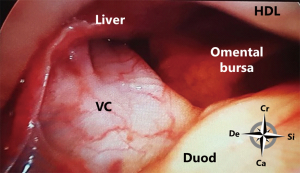
At the superior recess and from caudal to cranial the posterior wall consists of the inferior vena cava and the caudate lobe (segment 1) of the liver up to the right crus of the diaphragm. The anterior side of the hepatoduodenal ligament is located inside the omental bursa and forms the most caudal part of the superior recess (Figures 2 and 6). The peritoneal cavity and the omental bursa are connected through the omental foramen (foramen of Winslow). The boundaries of the omental foramen are as follows: it is bounded cranially by the caudate lobe, caudally by the first part of the duodenum, ventrally by the hepatoduodenal ligament, and dorsally by the inferior vena cava (Figure 7). In this way, both the anterior and posterior aspects of the hepatoduodenal ligament are covered by peritoneum of the omental bursa (Figure 6). After entering the omental foramen one enters the vestibulum of the omental bursa.
Bursectomy
The systematic review resulted in a total of 53 articles, of which 7 remained after screening title/abstract evaluating the effect of bursectomy during gastrectomy for cancer. These included several retrospective studies and 1 randomized controlled trial.
Bursectomy is associated with a prolonged operative time and increased blood loss, but not with increased postoperative morbidity (6). Regarding survival, retrospective series demonstrate ambiguous results (7-10). The only randomized controlled trial could not demonstrate a survival benefit of bursectomy, nor could it demonstrate non-inferiority of the omission of bursectomy (6).
The studies comparing bursectomy and non-bursectomy were all based on open surgical procedures. Only one study described a totally laparoscopic bursectomy and demonstrated it to be safe (11).
Discussion
During MIS of the upper GI tract, including esophageal, gastric and duodenopancreatic resection, the omental bursa may be a difficult area to visualize and to dissect when surgeons try to perform an adequate lymphadenectomy of the celiac trunk and branches and resection of the involved organ. It is a complex area in which during the ontogenesis the embryological anatomy developed into a crossroads of important vessels and digestive organs (1). According to Larsen, around 35 days the embryo’s developing stomach undergoes a 90-degree rotation around a craniocaudal axis so that the greater curvature lies to the left and the lesser curvature to the right (1). The rotation of the stomach and growth of the dorsal mesogastrium create a sac (the greater omentum) that dangles from the greater curvature of the stomach downward. When the duodenum swings to the right, it fuses to the posterior body wall (becoming secondarily retroperitoneal), enclosing the space posterior to the stomach and the space created by the greater omentum. This space is the omental bursa or lesser sac, the huge remainder of the peritoneal cavity is called the greater sac. The only passageway between the greater and lesser sacs is the omental foramen of Winslow.
MIS is increasingly being performed and certain interventions are being evaluated through randomized controlled trials (12-14). Moreover, robot-assisted MIS displaying improved ergonomy and 3-dimensional vision, increasingly aids in difficult dissections during determined phases of MIS and complicated reconstructive phases (15). The obvious advantages of MIS are its magnification and its optimal visualization. But, at the same time, we lose the benefit of having tactile feeling which is crucial for these complex procedures. Missing this tactile feeling can be compensated by having an optimal knowledge of every angle and perspective of the surgical anatomy, including the omental bursa and its boundaries.
In this observational study of upper abdominal MIS, our aim was to describe the surgical anatomical aspects of the omental bursa, with surgical landmarks and folds that have to be visualized and dissected, in order to perform an adequate lymphadenectomy followed by an oncological resection of the involved organ. Moreover, the two most common ways for surgically approaching the omental bursa, first through the lesser omentum and second through the gastrocolic ligament, are described. Both are not exclusive and in many events a combination of both approaches can help to achieve an adequate lymphadenectomy.
Remarkable is the description of three different folds, the right and left gastroomental folds and the short gastric vessels fold, containing the right and left gastroepiploic vessels and the short gastric vessels respectively. Knowledge of the configuration of these folds during laparoscopic gastrectomy is important during lymphadenectomy and gastric resection. In order to access the right gastroepiploic group (station 6) the right gastroomental fold should be visualized after tilting the stomach and dissected free from the mesocolon up to the pancreas. At this level, lymphadenectomy of this group and ligation and division of the vessels close to their origins (important during radical gastric resection) may be tedious, due to fat deposition which makes it difficult to differentiate fat from pancreatic parenchyma.
The configuration of the vessels is interesting and because of anatomical variations carefully dissection along the planes is mandatory. The conventionally described configuration of the celiac trunk and its branches occurs in only 55% of the population (16). It may be incomplete or branches may arise from other sources such as the right hepatic artery from the superior mesenteric artery (12%) or the left hepatic from the left gastric artery (25%). Moreover, branches of these three main branches show also important variations; thus replacing and accessory vessels are very frequent and should be taken into account during the dissection (17). Important in esophageal resection and gastric tube creation is the description by Koskas and Gayet of four types of anastomotic modes of the arterial gastroepiploic circle of the greater gastric curvature (18). Interruption of the circles, by anatomical variation or by dissection may produce inadvertent ischemia of the gastric tube leading to complications. CT angiography, performed nowadays more frequently should be carefully studied before surgery to anticipate those anatomical variations (19). Interesting is to know the gastrocolic vein of Henle and the variable course of the left gastroepiploic vein that runs in 30% of the patients in front of the common hepatic artery and in 70% behind it.
It is important to understand the location of the root of the transverse mesocolon reaching up to the lower edge of the body, tail and middle of the pancreas head. The hepatoduodenal ligament contains the hepatic artery proper along with the portal vein and the common bile duct and lies at the right boundary of the bursa. Lymph nodes at the hilum of the liver, group number 12a, are located at the edge of the omental bursa. Moreover, lymph node group 10 is also located outside the omental bursa at the splenic hilum. It is accessed after opening and dividing the left gastroomental fold.
In this study, in two patients the lymphadenectomy planned was considered not complete according to the Japanese Gastric Cancer Treatment Guidelines (4). Importantly, adequate lymphadenectomy should be considered as the most important quality control of this type of surgery.
Another important topic is whether a bursectomy should be considered necessary for invasive gastric cancer, stages T3 and T4a as advised by the Japanese Gastric Cancer Treatment Guidelines (4). In addition to the limited evidence, there are some questions with regard to the validity of this advice from the anatomic point of view. As the omental bursa is connected to the peritoneal cavity through the foramen of Winslow, it is unlikely that viable cancer cells disseminated into the bursa remain restricted to this cavity (20). In the Western world, bursectomy is only performed in selected patients since literature is scarce, as demonstrated by this study. The possibility of bursectomy during MIS was only demonstrated by one study (11).
In conclusion, it appears that dissection of structures surrounding the bursa by MIS can be demanding because of the complex anatomy (1). We have argued that having profound insight into the surgical anatomy and landmarks of the resection will enable a more adequate and reproducible surgical resection during upper GI MIS. In this respect, the pertaining comprehensive description is significant for three reasons. First, it defines the anatomical structures, boundaries and landmarks of the omental bursa. Second, it describes the two most frequent used approaches to the omental bursa in order to perform lymphadenectomy of the celiac trunk and branches, followed by resection of the involved organ. And third, it describes the steps to operate, pointing out how important it is to understand and dissect the marginal folds in order to dissect the lymph node groups located at the edge the omental bursa, the so called difficult stations 6, 10 and 12a. The conclusion can be that the advantages gained by MIS, such as visualization and magnification, contribute to a more complete knowledge of the omental bursa with its central location in the upper abdomen.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Larsen W. Development of the Gastrointestinal Tract. In: Sherman L, Potter S, Scott W. editors. Human Embryology. 3rd ed. Churchill Livingstone, 2001:235-64.
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [Crossref] [PubMed]
- Hirao M, Kurokawa Y, Fujita J, et al. Long-term outcomes after prophylactic bursectomy in patients with resectable gastric cancer: Final analysis of a multicenter randomized controlled trial. Surgery 2015;157:1099-105. [Crossref] [PubMed]
- Eom BW, Joo J, Kim YW, et al. Role of bursectomy for advanced gastric cancer: result of a case-control study from a large volume hospital. Eur J Surg Oncol 2013;39:1407-14. [Crossref] [PubMed]
- Kochi M, Fujii M, Kanamori N, Kaiga T, et al. D2 gastrectomy with versus without bursectomy for gastric cancer. Am J Clin Oncol 2014;37:222-6. [Crossref] [PubMed]
- Zhang WH, Chen XZ, Yang K, et al. Bursectomy and non-bursectomy D2 gastrectomy for advanced gastric cancer, initial experience from a single institution in China. World J Surg Oncol 2015;13:332. [Crossref] [PubMed]
- Yoshikawa T, Tsuburaya A, Kobayashi O, et al. Is bursectomy necessary for patients with gastric cancer invading the serosa? Hepatogastroenterology 2004;51:1524-6. [PubMed]
- Zou L, Xiong W, Mo D, et al. Totally laparoscopic complete bursectomy and D2 lymphadenectomy in radical total gastrectomy: an outside bursa omentalis approach. Surg Endosc 2016;30:4152. [Crossref] [PubMed]
- Wang M, Cai H, Meng L, et al. Minimally invasive pancreaticoduodenectomy: A comprehensive review. Int J Surg 2016;35:139-46. [Crossref] [PubMed]
- Haverkamp L, Brenkman HJ, Seesing MF, et al. Laparoscopic versus open gastrectomy for gastric cancer, a multicenter prospectively randomized controlled trial (LOGICA-trial). BMC Cancer 2015;15:556. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Surgical techniques, open versus minimally invasive gastrectomy after chemotherapy (STOMACH trial): study protocol for a randomized controlled trial. Trials 2015;16:123. [Crossref] [PubMed]
- van Hillegersberg R, Seesing MF, Brenkman HJ, et al. Robot-assisted minimally invasive esophagectomy. Chirurg 2017;88:7-11. [Crossref] [PubMed]
- Netter F. Blood supply of Stomach and Duodenum. Digestive System. Part 1. Upper Digestive Tract. Volume 3 ed. Ciba Pharmaceutical Company, 1959.
- Michels N. Blood supply and Anatomy of the Upper Abdominal Organs; With a Descriptive Atlas (172 illustrations). Philadelhia: JB Lipincott Company, 1954.
- Koskas F, Gayet B. Anatomical study of retrosternal gastric esophagoplasties. Anat Clin 1985;7:237-56. [Crossref] [PubMed]
- Sureka B, Mittal MK, Mittal A, et al. Variations of celiac axis, common hepatic artery and its branches in 600 patients. Indian J Radiol Imaging 2013;23:223-33. [Crossref] [PubMed]
- Yamamura Y, Ito S, Mochizuki Y, et al. Distribution of free cancer cells in the abdominal cavity suggests limitations of bursectomy as an essential component of radical surgery for gastric carcinoma. Gastric Cancer 2007;10:24-8. [Crossref] [PubMed]