Protein regulator of cytokinesis-1 expression: prognostic value in lung squamous cell carcinoma patients
Introduction
Lung cancer, as one of the most prolific malignancies, is the leading cause of cancer death. Lung cancer caused about 21.6% of all cancer-related deaths in 2015 in China (1). Small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) are two major histological types of lung cancers. The latter includes squamous cell carcinoma (SCC), adenocarcinoma (ADC), and large cell carcinoma (2). Lung SCC is the main type of NSCLC, and also the second-largest histologic lung cancer subtype. Even though treatment improves the patient’s prognosis, the 5-year overall survival (OS) rate of all lung cancer patients is 10–15% (3,4). The high rates of distant metastasis after resection may explain the dismal prognosis of NSCLC. Therefore, identification of biomarkers and new functional genes related to tumor development could provide alternative approaches for the treatment of those suffering from lung cancer.
Jiang et al. [1998] identified the gene for protein regulator of cytokinesis-1 (PRC1), which is considered a mitotic spindle associated cyclin dependent kinase (CDK) substrate (5). The PRC1 gene is located at 15q26.1, and it encodes a 620 amino acid protein with a molecular size of 71 dKa (5). The inhibition of endogenous PRC1 by siRNAS was shown to induce the dysregulation of mitosis and inhibited the process of cytokinesis, which results in two nuclei in the cells (5). In addition, accumulating evidence also suggests that PRC1 plays key roles in microtubule crosslinking (6,7).
PRC1 has been shown to overexpress in various cancers including breast cancer (8), bladder cancer (9), hepatocellular carcinoma (10,11) and pancreatic cancer (12). The previous study (13) showed that PRC1 was up-regulated in primary gastric cancers and overexpression of PRC1 in gastric cancers was associated with poor disease-specific survival and OS. Recently, Chen et al. (11) found that PRC1 had an oncogenic function in promoting cancer proliferation, metastasis and tumorigenesis of hepatocellular carcinoma. Currently, the expression profile of PRC1 and the prognostic value of PRC1 in lung SCC have not been determined.
Methods
Patients
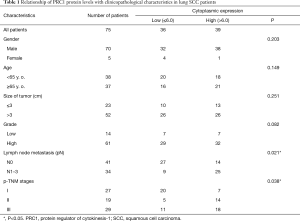
Commercial lung cancer tissue microarrays (TMAs) were obtained from the National Engineering Center for BioChips in Shanghai, China. All tissues were confirmed as lung SCC or adjacent normal lung tissue using histopathological methods. The clinicopathologic characteristics of the lung SCC patients are summarized in Table 1. The research protocol was reviewed and approved by the Ethical Committee and Institutional Review Board of the Nanjing University (2017NZHX-018), and written informed consent was obtained from each patient included in the study.
Full table
Immunohistochemical staining and scoring in TMAs
To evaluate PRC1 expression, 75 lung SCC samples and paired adjacent normal lung tissues were used in commercial TMAs (Hlug-squ150sur-01, Shanghai Outdo Biotech, Shanghai, China). Antigen retrieval was performed using high pressure about 5 minutes and citrate buffer for pH 6.0. Sections were incubated with the primary anti-PRC1 antibody for overnight at 4 °C (Rabbit monoclonal anti-PRC1 antibody, ab51248, Abcam). PBS replacement of the primary antibody was used for negative control analysis. The samples were then independently analyzed by two pathologists who did not have any clinical information about the patients. The scores for protein expression were determined as previously described (14,15). Staining intensity was scored as the following: 0 (negative), 1 (weak), 2 (medium) or 3 (strong). The level of staining was taken as the percentage of areas with positive stained tumor cells in relation to the entire tumor area. Areas were scored using the following scale: 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%) and 4 (76–100%). Multiplying the positivity and intensity scores gave the total score of PRC1 protein expression (overall scores, 12).
Statistical analysis
GraphPad Prism 6.00 (GraphPad Software, La Jolla California, USA) was used for all statistical analyses. A paired t-test was performed to determine the difference in the level of protein expression of PRC1 in lung SCC and adjacent matched normal tissues. A Mann-Whitney U-test was performed to assess associations between PRC1 expression and the patient clinicopathological characteristics. The day of surgery to the date of the last follow-up or the date of death was considered the OS value and survival at the last follow-up was censored. The Kaplan-Meier method was used to calculate the post-operative survival curves. A log-rank test was used to determine differences in the survival rates. Statistical significance was set at P<0.05.
Results
PRC1 is upregulated in lung SCC tissues
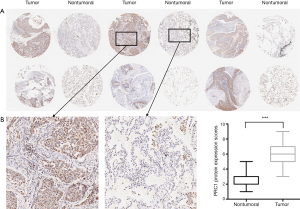
We first examined PRC1 protein levels and distribution in 75 pairs of lung SCC tissues and matched adjacent normal tissues using immunohistochemical staining (Figure 1A). PRC1 protein was predominantly located in cytoplasm. The scores of PRC1 protein expression were calculated by multiplying the positivity and intensity scores. Cytoplasmic PRC1 protein levels were also higher in the 75 lung SCC tissues than in the matched adjacent normal tissues (P<0.001), as shown in Figure 1. These data showed PRC1 expression is up-regulated in lung SCC tissues.
Correlations between PRC1 levels and clinicopathological factors in lung SCC patients
The 75 lung SCC patients were broken into two groups according to mean expression level (staining scores) to ascertain the clinical importance of cytoplasmic PRC1 levels. Correlations between the clinicopathological factors and PRC1 levels in patients with lung SCC are shown in Table 1. Cytoplasmic PRC1 staining scores of ≤6 were defined as low level and scores >6 were defined as high level (Figure 2A). High cytoplasmic PRC1 levels were associated with tumor size (P=0.021) and p-TNM stage (P=0.038). There was no significant association between gender, age, size of tumor, grade and PRC1 expression.
PRC1 contributes to poor prognosis in lung SCC patients
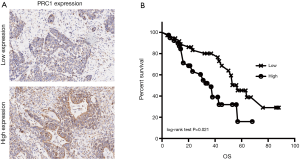
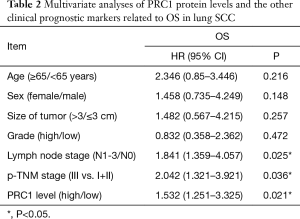
Seventy-five patients were broken into two groups according to the mean expression level to ascertain the importance of PRC1 overexpression in predicting lung SCC clinical outcomes. It is interesting that patients with higher levels of PRC1 protein had a shorter survival time than patients with lower PRC1 levels (P=0.021, log-rank test, Figure 2B). Notably, multivariate Cox regression analysis showed that N stage, p-TNM Stage and high expression of PRC1 protein were independent prognostic factors for lung SCC patients (Table 2).
Full table
Discussion
Lung SCC is a prevalent subtype of NSCLC that is often fatal. SCC is mainly diagnosed in people with a history of smoking and is responsible for approximately 20–30% of new lung cancer cases (1). Until recently, the various subtypes of NSCLC were grouped together for the purpose of treatment, but it is now widely known that different histologic subtypes should be treated as separate disease entities (16). SCC is the second most prevalent type of lung cancer. However, because the actionable gene mutations of this disease are not well understood, no approved targeted therapeutics is available for its treatment up to date (17). Therefore, performing comparative analyses of lung SCCs and identifying potential therapeutic targets would lead to significant growth in cancer treatments.
The PRC1 gene was initially thought to be a CDK substrate according to an in vitro phosphorylation screen. It was subsequently shown to be a midzone-associated protein that was required for completion of cytokinesis (5). PRC1 is phosphorylated by CDK1 (Cdc2/Cyclin B) in early mitosis. PRC1 is then changed to an inactive and monomeric state (6). It is dephosphorylated during the metaphase-anaphase transition and interacts with KIF4, a kinesin motor that translocates PRC1 towards the plus end of antiparallel interdigitating microtubules along mitotic spindles. The dephosphorylated PRC1 protein parcels the antiparallel interdigitating microtubules to establish the midzone, which is necessary for cytokinesis (18).
Cytokinesis is a complex process, involving sophisticated biochemical and cellular dynamics factors. Abnormal cytokinesis has been shown to lead to cell chromosome instability and malignant transformation (19,20). Dysregulated expression of PRC1, as a critical protein of cytokinesis, can cause disorders of cytokinesis. Previous studies (8,9,11) have implicated PRC1 as an oncogenic factor in tumorigenesis of bladder cancer, breast, and hepatocellular carcinoma. Here, we showed that PRC1protein expression was upregulated in lung SCC compared to paired normal lung tissues. We then found overexpression of PRC1 protein associated with metastasis of lymph node and was an independent poor prognostic marker for lung SCC patients.
There is sufficient evidence that p53 enhances the sensitivity of tumor cells to chemotherapy and radiotherapy, induces cell cycle arrest and inhibits cell growth and the proliferation of tumor cells (21-23). Li et al. (24) showed that p53 directly suppresses PRC1 gene transcription. In this study, they showed p53 interacts with the PRC1 gene promoter in breast cancer cells. This indicated that PRC1 is a common downstream target of p53. PRC1 was also confirmed as a downstream target of heat shock transcription factor 2 (HSF2) and is involved regulating cytokinesis (25). Moreover, our previous study (13) indicated that knockdown expression of PRC1 suppressed the proliferation and invasion of lung gastric carcinoma in vitro and in vivo. Collectively, these results indicate that silencing of PRC1 could inhibit tumorigenesis by blocking cytokinesis in a wide assortment of cancer cells. PRC1 is expected to become a new target for antitumor gene therapy.
In summary, we first identified that the expression pattern and the clinicopathological characteristics of PRC1 in lung SCC. Our results supply a foundation for the concept that PRC1 is upregulated in human SCC tissues and overexpression of PRC1 in human lung SCC could be vital in the attainment of an aggressive and poor prognostic phenotype. Further study is required to achieve a full understanding of the underlying molecular mechanisms.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81302032, 81401903, 81572937 and 81572273), the Natural Science Foundation of Jiangsu province (No. BK2011658, BL2013026 and BK20140736), Program of Nanjing Science and Technology of Nanjing Science and Technology Committee (No. 201605059) and Jiangsu Provincial Medical Youth Talent (No. QNRC2016125). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The research protocol was reviewed and approved by the Ethical Committee and Institutional Review Board of the Nanjing University (2017NZHX-018), and written informed consent was obtained from each patient included in the study.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA-Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. Non-Small Cell Lung Cancer, Version 6. J Natl Compr Canc Netw 2015;13:515-24. [Crossref] [PubMed]
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin 2012;62:220-41. [Crossref] [PubMed]
- 4 Foley EA, Kapoor TM. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol 2013;14:25-37. [PubMed]
- Jiang W, Jimenez G, Wells NJ, et al. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol Cell 1998;2:877-85. [Crossref] [PubMed]
- Mollinari C, Kleman JP, Jiang W, et al. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J Cell Biol 2002;157:1175-86. [Crossref] [PubMed]
- Subramanian R, Wilson-Kubalek EM, Arthur CP, et al. Insights into antiparallel microtubule crosslinking by PRC1, a conserved nonmotor microtubule binding protein. Cell 2010;142:433-43. [Crossref] [PubMed]
- Shimo A, Nishidate T, Ohta T, et al. Elevated expression of protein regulator of cytokinesis 1, involved in the growth of breast cancer cells. Cancer Sci 2007;98:174-81. [Crossref] [PubMed]
- Kanehira M, Katagiri T, Shimo A, et al. Oncogenic role of MPHOSPH1, a cancer-testis antigen specific to human bladder cancer. Cancer Res 2007;67:3276-85. [Crossref] [PubMed]
- Wang SM, Ooi LL, Hui KM. Upregulation of Rac GTPase-activating protein 1 is significantly associated with the early recurrence of human hepatocellular carcinoma. Clin Cancer Res 2011;17:6040-51. [Crossref] [PubMed]
- Chen J, Rajasekaran M, Xia H, et al. The microtubule-associated protein PRC1 promotes early recurrence of hepatocellular carcinoma in association with the Wnt/β-catenin signalling pathway. Gut 2016;65:1522-34. [Crossref] [PubMed]
- Nakamura T, Furukawa Y, Nakagawa H, et al. Genome-wide cDNA microarray analysis of gene expression profiles in pancreatic cancers using populations of tumor cells and normal ductal epithelial cells selected for purity by laser microdissection. Oncogene 2004;23:2385-400. [Crossref] [PubMed]
- Zhang B, Shi X, Xu G, et al. Elevated PRC1 in gastric carcinoma exerts oncogenic function and is targeted by piperlongumine in a p53-dependent manner. J Cell Mol Med 2017. [Epub ahead of print]. [PubMed]
- Zhan P, Lv XJ, Ji YN, et al. Increased lysyl oxidase-like 2 associates with a poor prognosis in non-small cell lung cancer. Clin Respir J 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Liu H, Wu Y, Zhu S, et al. PTP1B promotes cell proliferation and metastasis through activating src and ERK1/2 in non-small cell lung cancer. Cancer Lett 2015;359:218-25. [Crossref] [PubMed]
- Langer CJ, Besse B, Gualberto A, et al. The evolving role of histology in the management of advanced non-small-cell lung cancer. J Clin Oncol 2010;28:5311-20. [Crossref] [PubMed]
- Kim Y, Hammerman PS, Kim J, et al. Integrative and comparative genomic analysis of lung squamous cell carcinomas in East Asian patients. J Clin Oncol 2014;32:121-8. [Crossref] [PubMed]
- Zhu C, Lau E, Schwarzenbacher R, et al. Spatiotemporal control of spindle midzone formation by PRC1 in human cells. Proc Natl Acad Sci USA 2006;103:6196-201. [Crossref] [PubMed]
- Foley EA, Kapoor TM. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol 2013;14:25-37. [Crossref] [PubMed]
- Doominguez-Brauer C, Thu KL, Mason JM, et al. Targeting Mitosis in Cancer: Emerging Strategies. Mol Cell 2015;60:524-36. [Crossref] [PubMed]
- Khoo KH, Verma CS, Lane DP. Drugging the p53 pathway: understanding the route to clinical efficacy. Nat Rev Drug Discov 2014;13:217-36. [Crossref] [PubMed]
- Zhang S, Zhou L, Hong B, et al. Small-Molecule NSC59984 Restores p53 Pathway Signaling and Antitumor Effects against Colorectal Cancer via p73 Activation and Degradation of Mutant p53. Cancer Res 2015;75:3842-52. [Crossref] [PubMed]
- Guo XL, Hu F, Zhang SS, et al. Inhibition of p53 increases chemosensitivity to 5-FU in nutrient-deprived hepatocarcinoma cells by suppressing autophagy. Cancer Lett 2014;346:278-84. [Crossref] [PubMed]
- Li C, Lin M, Liu J. Identification of PRC1 as the p53 target gene uncovers a novel function of p53 in the regulation of cytokinesis. Oncogene 2004;23:9336-47. [Crossref] [PubMed]
- Murphy LA, Wilkerson DC, Hong Y, et al. PRC1 associates with the hsp70i promoter and interacts with HSF2 during mitosis. Exp Cell Res 2008;314:2224-30. [Crossref] [PubMed]


