Effect of Huisheng oral solution on coagulation function in perioperative period in patients with primary lung cancer
Introduction
The relationship between malignant tumors and venous thromboembolism (VTE) has been a hotspot in the malignant tumor treatment field. VTE, including deep vein thrombosis (DVT) and pulmonary embolism (PE), is one of the major complications of malignant tumors (1). The mortality rate of lung cancer has increased significantly in China. According to the third national retrospective sampling survey of death causes conducted by the Ministry of Health of China, lung cancer has become the first cause of cancer death among Chinese (2). Patients with lung cancer are at a high risk for VTE. According to reports in the literature, the incidence of VTE is as high as 13.6% in patients with non-small cell lung cancer (3). Current main treatments for cancer include surgery, chemotherapy, radiotherapy, and targeted therapy. All these treatments may increase the risk of VTE in patients (4-11). In recent years, researchers have attempted to study the correlation between lung cancer and abnormal coagulation as well as the prevention and treatment of abnormal coagulation in lung cancer patients. According to the recommendations for VTE prophylaxis and treatment in patients with cancer issued by American Society of Clinical Oncology in the year of 2007, cancer patients, especially those undergoing surgery, should be considered for VTE prophylaxis with anticoagulants. Anticoagulant therapy should be carried out preoperatively and re-administered postoperatively as soon as possible for patients without bleeding risk. The treatment should last at least 7–10 days, and it should be extended to a month for high-risk patients (12,13). At present, there has been no consensus on perioperative anticoagulation in lung cancer patients, and low molecular heparin is the main drug currently used for the prevention and treatment of VTE. Although low molecular heparin has the advantages of low bleeding risk and good anticoagulant effect (14), it is administered by subcutaneous injection. After patients are discharged from hospital, their compliance to heparin treatment will significantly reduce. The clinical effects of oral medications including rivaroxaban, dabigatran and apixaban still need further verification (15-17).
The effect of oral traditional Chinese medicine preparations in the prevention and treatment of thromboembolic diseases has attracted wide attention. A large number of Chinese herbals can promote blood circulation and remove blood stasis, such as safflower, leech, rhizoma ligustici wallichii, and notoginseng. They have been demonstrated pharmacologically and clinically to reduce platelet aggregation or blood viscosity and have a clear anticoagulant and antithrombotic effect (18-21). Huisheng oral solution (HSOS) is a traditional Chinese medicine preparation for the treatment of lung cancer. An important characteristic of the HSOS recipe lies in that it contains a large proportion of herbals that can promote blood circulation and remove blood stasis, such as rhizoma ligustici wallichii, safflower, leech, cattail pollen, and trogopterus dung. HSOS has been used clinically for many years. In a recent basic study on the effect of blood circulation-promoting mechanism on tumor treatment, HSOS was demonstrated that it could effectively inhibit carageenan induced acute thrombosis in rats, decrease the abnormal increase of platelets and reduce blood fibrinogen degradation product (FDP) concentrations (22). In another study, HSOS could reduce the incidence of thrombosis in the lungs and mesentery of tumor-bearing mice, and inhibit tumor cell metastasis (23). However, the anticoagulant effect of HSOS in the perioperative period in patients with lung cancer is still unclear.
Methods
Patients
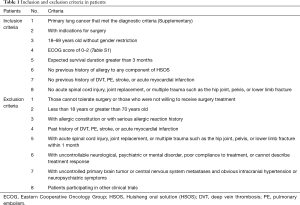
All patients had pathologically confirmed lung cancer. The inclusion and exclusion criteria in patients were shown in Table 1.
Full table
All patients underwent surgical treatment at Department of Thoracic Surgery of the Fourth Hospital of Hebei Medical University (also named as Hebei Provincial Tumor Hospital), the Second Hospital of Hebei Medical University, Handan Central Hospital, Cangzhou Central Hospital, or Affiliated Hospital of North China Coal Medical University from March 2013 to May 2015. All the hospitals were tertiary referral hospitals, of which two were located in the central part of Hebei Province, one in the south, one in the east, and one in the north. Three of these hospitals were Affiliated Hospitals of Hebei Medical University. The type of surgery which patients underwent was shown in Table 2. This study was approved by the local ethics committee, and all patients provided written informed consent. This study was registered in the Chinese Clinical Trial Registry (ChiCTR-TRC-13003325).

Full table
Study design
This study was a multicenter, randomized, single-blind, blank-controlled clinical trial. The study protocol and implementation manual were developed by the Fourth Hospital of Hebei Medical University and approved by the local ethics committee. After obtaining approval, the staff, including responsible clinicians, nurses and medical laboratory technicians at other centers who were involved in this study were trained.
Given a two-sided type I error of 0.05, a type II error of 0.1, a dropout rate of 10%, and the allocation ratio of 1:1, a total number of sample size of 160 patients (80 in each group) was determined.
The patients were enrolled from March 2013 to March 2015. Each center was allocated 30 patients with exception of the Fourth Hospital of Hebei Medical University (40 patients).
Patients at each center were randomized to either a study group or a control group by random number table. The randomization code was stored in a sealed envelope, which would be open twice for unblinding. Since all outcome measures of this study were blood biochemical parameters, the investigators and subjects were not blinded to the examinations. Blood samples were marked with the patient’s name, center and medical record number, time at which the sample was taken, and times of sample taking, but the group the patient belonged to was not marked.
The study group was given oral HSOS (20 mL, bid) until 24 h surgery. If no active bleeding was noted, the patients were given oral HSOS (20 mL, tid) from 24 h to 24 d postoperatively. During the study period, the patients in the study group did not receive any other anticoagulation therapy. The control group only underwent surgery and did not receive any anticoagulation therapy.
Outcome evaluation
Blood samples were taken at admission (before therapy), 24 h, 72 h, 10 d (before discharge) and 24 d (first visit after discharge) after surgery. Routine blood tests [red blood cell (RBC) count, white blood cell (WBC) count, hemoglobin (HGB), and platelet (PLT) count] and coagulation function test [prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen (FIB), and plasma D-dimer] were performed.
Statistical analysis
All statistical analyses were performed using SPSS13.0 software. Numerical data are expressed as mean ± standard deviation (SD), and categorical data are expressed as percentages. Numerical data that follow a normal distribution and have homogeneous variance were compared using t-tests, otherwise they were compared using nonparametric tests. Categorical data were compared using χ2 tests. The changes in outcome measures over time were analyzed by repeated measures analysis of variance to compare the differences between groups and between different time points and assess the impact of tumor stage and mode of surgery on them. All tests were two-tailed, and P values <0.05 were considered statistically significant.
Results
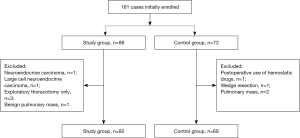
From March 2013 to March 2015, a total of 161 patients were enrolled. Ten patients were excluded due to undergoing exploratory thoracotomy only, undergoing wedge resection, with pulmonary mass that was not pathologically confirmed as lung cancer, with neuroendocrine carcinoma, with large cell neuroendocrine carcinoma, or postoperative use of hemostatic drugs. Finally, 151 patients were included. The patient enrollment flowchart is shown in Figure 1.
General clinical data
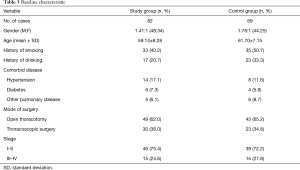
Baseline characteristic included gender, age, history of smoking and drinking, height, body weight, comorbid diseases, surgical procedure and tumor stage. There was no significant difference in these data between two groups (Table 3).
Full table
Repeated measures analysis of variance
Outcome measures followed a normal distribution and were analyzed by repeated measures analysis of variance except D-dimer. The data were stratified by gender, smoking status, drinking status, hypertension status, diabetes status, surgical procedure, and tumor stage to assess the impact of these factors on outcome measures.
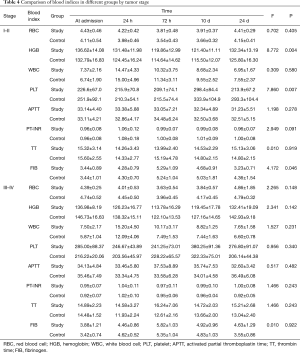
With regard to tumor stage, there were significant differences in some indices between the two groups. In patients with stage I–II disease, there were significant differences in HGB, PLT and FIB between the two groups, although no significant differences were observed in patients with stage III–IV disease (Table 4).
Full table
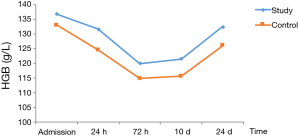
In patients with stage I–II disease, HGB decreased postoperatively, reached the lowest level at 72 h, and then gradually increased. At 72 h and 10 d postoperatively, HGB was significantly lower in the control group than in the study group (Table 5, Figure 2).

Full table
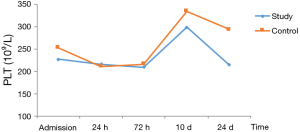
In patients with stage I–II disease in both groups, PLT decreased postoperatively, but reached the highest level at 10 d, and then decreased at 14 d. The decrease in PLT differed significantly between the two groups (Table 6, Figure 3).
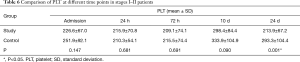
Full table
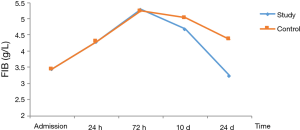
In patients with stage I–II disease, change of FIB was slightly different from those of PLT. FIB reached the peak at 72 h, and then decreased. The decrease in FIB to the normal range was significantly faster in the study group than in the control group (Table 7, Figure 4).
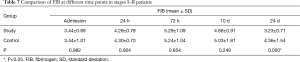
Full table
In patients with stage I–II disease, D-dimer had a skewed distribution and rank-sum test was therefore used for analysis. There was a significant difference in D-dimer at 24 d between the two groups (Table 8, Figure 5).

Full table
We have paid great attention to the occurrence of VTE and bleeding. Those patients with possible syndrome as VTE after surgery, such as dyspnea, massive hemoptysis, received other test like MRI or CT. VTE was not observed in both groups. Only one patients in the control group was observed with bleeding and there was no statistical significance between two groups (P=0.457).
Discussion
On the basis of previous research results, this study further assessed the short-term anticoagulation effect of HSOS in the perioperative period in patients with primary lung cancer. This study was led by Hebei Provincial Tumor Hospital, and the other hospitals involved are teaching tertiary referral centers located in the east, south, north and middle parts of Hebei Province, respectively, which can well reflect the lung cancer patient situation and treatment in China.
According to the recommendations for VTE prophylaxis and treatment in patients with cancer issued by ASCO in the year of 2007, it has been around 10 years since the study of malignant cancer, blood coagulation dysfunction and anticoagulation therapy in both animals and patients. From reviews published by Cochrane collaboration center (24-26), several conclusions could be reached. Firstly, it is clear that anticoagulation therapy for cancer could reduce the incidence of VTE, while whether it could have long-term effect of survival is unclear. Secondly, caner and blood hypercoagulability correlates with each other. Inflammatory reaction, vascular endothelial injury, coagulation initiation, platelet activation, tumor cell adhesion and aggregation are involved in this process, with a numerous number of cytokines and proteins (27-37). Take platelet activation for example, though it has been demonstrated to correlate with tumor metastasis (38-40), it is rare to detect the plate activation relevant cytokines. Common coagulation indices are mainly used to monitor the bleeding risk of vitamin K antagonists. However, they could not be used as potential indices for effect of coagulation for cancer patients. Thirdly, studies have revealed that aspirin could improve the survival of several cancers, which is quite different with heparin (41-43). Aspirin has the effect of anti-inflammatory and inhibition the activation and aggregation of platelet. Further studies could be conducted to reveal whether aspirin has more effect on cancer patients. Fourthly, current studies do not have enough power to distinguish different tumor types, different development periods, different drugs, and different test indices. More precise and systematic studies should be conducted.
This study found that the blood coagulation indices in lung cancer patients peaked 7 to 10 d after surgery and declined a month after surgery. This result is inconsistent with the previous finding that the hypercoagulation state peaked 3 d after surgery (44). This difference may be caused by different types of diseases. Since the mean hospitalization duration for Chinese patients who underwent surgery for lung cancer is 7–10 days, our finding suggests that the coagulation risk in many patients peaked when they were discharged from hospital and therefore cannot receive good hospital monitoring. This also confirms the necessity of sufficient and timely anticoagulation treatment. Treatment with HSOS can obviously reduce blood coagulation indices 1 week and 1 month after surgery. Tumor stage had an impact on the results, and stage III–IV patients showed no difference in the indices examined between the two groups, suggesting that HSOS had a better effect in stage I–II patients. Whether there is a dose relationship remains to be studied.
The coagulation indices selected in this study included PLT, four blood coagulation indices, and D-dimer content. We chose these indices based mainly on the considerations of representativeness, feasibility, and practicality. In the process of malignant tumor progression or treatment, many biochemical indices can be used to assess coagulation status, such as clotting factors, platelet activation related factors, inflammatory mediators, coagulant substances produced by tumors, tissue factor, and anticoagulant related proteins (27-37). Choosing appropriate indices for evaluating VTE is of clinical importance. This study chose three types of indices, namely, PLT, coagulation time, and products of thrombosis, to reflect the possibility of hypercoagulation status, and these indices have certain representativeness. According to reports in the literature (45,46), PLT, plasma D-dimer content, and plasma FIB are closely related to the occurrence, development and prognosis of ovarian cancer, and abnormality of one or more of these indices has a remarkable influence on progression free survival and overall survival. These three indices are very important. In addition, in terms of feasibility and practicality, these three indices have long been used clinically and could be detected in all hospitals involved. Therefore, the quality control can be ensured. More importantly, these three indices are covered by China medical insurance, which can reduce the burden of patients and promote their wide use.
This study considered the influence of the following factors: tumor stage, mode of surgery, smoking, diabetes, and lymph node metastasis. Mode of surgery may have an impact on bleeding volume (47) and thus affect coagulation status. In this study, the patients enrolled underwent either open surgery or thoracoscopic surgery for lobectomy or pneumonectomy, and it was found that mode of surgery had no significant effect on the results. Among the above influencing factors assessed, tumor stage had the most significant impact on the results. In both the control group and study group, patients with stage III–IV disease developed hypercoagulation state more frequently than those with stage I–II disease, and this is especially obvious in PLT and FIB, which is consistent with the results of a previous study (48). HSOS showed variable efficacy in patients with different stages of disease. HSOS can significantly improve PLT, FIB, and D-dimer in patients with stage I–II disease, but its effect in patients with stage III–IV disease had no significant difference between the two groups. The dose used in this study may be insufficient in patients with stage III–IV disease.
HSOS did not increase the risk of perioperative bleeding. In this study, only one case of postoperative bleeding was observed in the control group, who discontinued the study after the use of hemostatic drugs. A patient in the study group developed vomiting after taking HSOS and discontinued the use of HSOS. Previous studies have reported a few cases of gastrointestinal reaction associated with HSOS use, mainly because ferula asafetida, a component of HSOS, has a special smell and may cause nausea and vomiting, which often disappeared after drug withdrawal (49,50). No other adverse reactions were reported.
HSOS has 34 formulations, including 22 formulations for activating blood circulation to dissipate blood stasis, for example, ligusticum wallichii, carthamus tinctorious, leeches, cattail pollen, excrement pteropus, etc. In addition to the animal studies (22,23), HSOS has also been shown to be effective in anticoagulation and survival for lung cancer patients with chemotherapy (51,52). As a safe and effective oral drug, HSOS has the following advantages: (I) it can overcome the drawback of bad compliance of low molecular heparin; (II) it cost only 8.40 to 16.80 Yuan daily for patients in Hebei Province. Compared with other currently available oral anticoagulants, HSOS is relatively cheap and covered by China medical insurance, which makes it suitable for Chinese patients.
This study has several limitations including the lack of more precise coagulation indices, short observation period, and no assessment of the impact of changes of coagulation indices on prognosis. These issues will be addressed in further studies.
In conclusion, HSOS (20 mL, tid) is of good safety profile and does not increase the risk of bleeding. With its unique characteristic of convenience for being taken, HSOS (20 mL, tid) could be a proper treatment for lung cancer patients in the perioperative period.
Primary lung cancer that met the diagnostic criteria
Diagnosis
According to the defection of primary bronchogenic lung cancer in the new edition of standard for diagnosis and treatment of common malignant cancers published by Chinese anticancer association:
- Medical history and symptom: with or without symptom at the early stage, ongoing cough, bloody sputum, chest pain, short breath, fever, or more than 40 years old, especially male, with long-term smoking history, progressive emaciation;
- Sign: diminished or suppressed respiration, pleural effusion, Horner’s syndrome, superficial lymphadenopathy could be touched at the later stage, anemia;
- Imaging feature: tumor diagnosed by X-ray, CT or MRI;
- Pathology: biopsy diagnosis by fiberbronchoscope, operative pathology or cytology, superficial lymphadenopathy.
Stage
According to the definition of TNM and stage by AJCC/UICC (7th edition) published in 2009.
Primary tumor (T)
- Tx: primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy.
- T0: no evidence of primary tumor.
- Tis: carcinoma in situ.
- T1: tumor ≤3 cm in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e., not in the main bronchus).
- T1a: tumor ≤2 cm in greatest dimension.
- T1b: tumor >2 cm but ≤3 cm in greatest dimension.
- T2: tumor >3 and ≤7 cm or tumor with any of the following features—involves the main bronchus, ≥2 cm distal to the carina, invades the visceral pleura, associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung.
- T2a: tumor >3 but ≤5 cm in greatest dimension.
- T2b: tumor >5 but ≤7 cm in greatest dimension.
- T3: tumor >7 cm or one that directly invades any of the following: chest wall (including superior sulcus tumors), diaphragm, mediastinal pleura, parietal pericardium; or tumor in the main bronchus <2 cm distal to the carina but without involvement of the carina; or associated atelectasis or obstructive pneumonitis of the entire lung or separate tumor nodule(s) in the same lobe.
- T4: tumor of any size that invades any of the following: mediastinum, heart, great vessels, trachea, esophagus, vertebral body, carina; separate tumor nodule(s) in a different ipsilateral lobe.
Regional lymph nodes (N)
- Nx: regional lymph nodes cannot be assessed.
- N0: no regional lymph node metastasis.
- N1: metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension.
- N2: metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s).
- N3: metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s).
Distant metastasis (M)
- Mx: distant metastasis cannot be assessed.
- M0: no distant metastasis.
- M1a: separate tumor nodule(s) in a contralateral lobe; tumor with pleural nodules or malignant pleural (or pericardial) effusion.
- M1b: distant metastasis.
Note: the uncommon superficial tumor of any size with its invasive component limited to the bronchial wall, which may extend proximal to the main bronchus, is also classified as T1.
Most pleural (and pericardial) effusions with lung cancer are due to tumor. In a few patients, however, multiple cytopathologic examinations of pleural (pericardial) fluid are negative for tumor, and the fluid is non-bloody and is not an exudate. Where these elements and clinical judgment dictate that the effusion is not related to the tumor, the effusion should be excluded as a staging element.

Full table
Acknowledgements
This study was supported by fund from Chengdu Diao Tianfu Pharmaceutical Group Co., LTD.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethics Statement: The study was approved by institutional ethics board of Fourth Hospital of Hebei Medical University (No. 2013012) and written informed consent was obtained from all patients.
References
- Farge D, Durant C, Villiers S, et al. Lessons from French National Guidelines on the treatment of venous thrombosis and central venous catheter thrombosis in cancer patients. Thromb Res 2010;125:S108-116. [Crossref] [PubMed]
- The Ministry of Health of the People’s Republic of China. Third national retrospect spot-check of death-causation. Beijing: Peking Union Medical College Press, 2008.
- Tagalakis V, Levi D, Agulnik JS, et al. High risk of deep vein thrombosis in patients with non-small cell lung cancer: a cohort study of 493 patients. J Thorac Oncol 2007;2:729-34. [Crossref] [PubMed]
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160:809-15. [Crossref] [PubMed]
- Cavo M, Zamagni E, Cellini C, et al. Deep-vein thrombosis in patients with multiple myeloma receiving first-line thalidomide-dexamethasone therapy. Blood 2002;100:2272-3. [Crossref] [PubMed]
- Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003;21:60-5. [Crossref] [PubMed]
- Kuenen BC, Levi M, Meijers JC, et al. Potential role of platelets in endothelial damage observed during treatment with cisplatin, gemcitabine, and the angiogenesis inhibitor SU5416. J Clin Oncol 2003;21:2192-8. [Crossref] [PubMed]
- Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 2007;99:1232-9. [Crossref] [PubMed]
- Vitale C, D’Amoto M, Calabro P, et al. Venous thromboembolism and lung cancer: a review. Multidiscip Respir Med 2015;10:28. [Crossref] [PubMed]
- Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 2008;300:2277-85. [Crossref] [PubMed]
- Zangari M, Flink LM, Elice F, et al. Thrombotic events in patients with cancer receiving antiangiogenesis agents. J Clin Oncol 2009;27:4865-73. [Crossref] [PubMed]
- Lyman GH, Khorana AA, Falanga A, et al. American society of clinical oncology guideline: Recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007;25:5490-505. [Crossref] [PubMed]
- Lyman GH, Kuderer NM. Prevention and treatment of venous thromboembolism among patients with cancer: The American society of clinical oncology guidelines. Thromb Res 2010;125:S120-7. [Crossref] [PubMed]
- Conti S, Guercini F, Lorio A. Low-Molecular-Weight Heparin and cancer survival: Review of the Literature and Pooled analysis of 1726 patients treated for at least three months. Pathophysiol Haemost Thromb 2003;33:197-201. [Crossref] [PubMed]
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52. [Crossref] [PubMed]
- EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499-510. [Crossref] [PubMed]
- Cohen AT, Spiro TE, Buller HR, et al. Extended-duration rivaroxaban thromboprophylaxis in acutely ill medical patients: MAGELLAN study protocol. J Thromb Thrombolysis 2011;31:407-16. [Crossref] [PubMed]
- Li SJ, Li WX, Tang YP, et al. Comparative analysis of the promoting blood effects of the combination of different proportions of Danggui and Honghua by the principal component analysis and multi-attribute comprehensive index methods. Yao Xue Xue Bao 2014;49:1304-9. [PubMed]
- Guo XQ, Sun JM, Zhang H. Chemical composition and pharmacological effects of leech. Jilin Journal of Traditional Chinese Medicine 2015;1:52-5.
- Jin YQ, Hong YL, Li JR, et al. Advances in research of chemical components and pharmacological effects of rhizoma ligustici wallichii. Pharmacy and Clinics of Chinese Materia Medica 2013;3:48-52.
- Xiao XF, Qu LH, Zheng M, et al. "Huoxuehuayu" effects of Herba Epimedii combined with Radix Notoginseng extracts combination and effects of the combination on passive avoidance of amnestic mice model induced by scopolamine. Chinese Journal of Modern Applied Pharmacy 2006;5:7-9.
- Liu SQ, Guo JY, Du J, et al. Anticoagulant effect of Huisheng oral solution in a rat model of thrombosis. Indian J Pharmacol 2013;45:359-64. [Crossref] [PubMed]
- Wang W, Wang H, Wang CM, et al. Treatment with Huisheng oral solution inhibits the development of pulmonary thromboembolism and metastasis in mice with Lewis lung carcinoma. Oncol Lett 2014;7:87-94. [PubMed]
- Akl EA, Kahale L, Terrenato I, et al. Oral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst Rev 2014.CD006466. [PubMed]
- Akl EA, Gunukula S, Barba M, et al. Parenteral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst Rev 2011.CD006652. [PubMed]
- Di Nisio M, Porreca E, Otten HM, et al. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev 2014.CD008500. [PubMed]
- Dicke C, Amirkhosravi A, Spath B, et al. Tissue factor-dependent and –independent pathways of systemic coagulation activation in acute myeloid leukemia: a single-center cohort study. Exp Hematol Oncol 2015;4:22. [Crossref] [PubMed]
- Wojtukiewicz MZ, Sierko E, Zacharski LR, et al. Tissue factor-dependent coagulation activation and impaired fibrinolysis in situ in gastric cancer. Semin Thromb Hemost 2003;29:291-300. [Crossref] [PubMed]
- Ozturk M, Sengul N, Dagli M, et al. Global fibrinolytic capacity in colorectal cancer: a new clue to occult fibrinolysis. Clin Appl Thromb Hemost 2003;9:151-4. [Crossref] [PubMed]
- Spek CA, Arruda VR. The protein C pathway in cancer metastasis. Thromb Res 2012;129 Suppl 1:S80-4. [Crossref] [PubMed]
- Kelly J, Rudd A, Lewis RR, et al. Plasma D-dimers in the diagnosis of venous thromboembolism. Arch Intern Med 2002;162:747-56. [Crossref] [PubMed]
- Sierko E, Wojtukiewicz MZ, Zawadzki R, et al. Expression of protein C(PC), protein S(PS) and thrombomodulin (TM) in human colorectal cancer. Thromb Res 2010;125:e71-5. [Crossref] [PubMed]
- Nash GF, Turner LF, Scully MF, et al. Platelets and cancer. Lancet Oncol 2002;3:425-30. [Crossref] [PubMed]
- Totan M, Dagdemir A, Ak AR, et al. Effects of high-dose methotrexate on the hemostatic system in childhood acute lymphoblastic leukemia. Med Pediatr Oncol 2001;36:429-33. [Crossref] [PubMed]
- Verheul HM, Hoekman K, Lupu F, et al. Platelet and coagulation activation with vascular endothelial growth factor generation in soft tissue sarcomas. Clin Cancer Res 2000;6:166-71. [PubMed]
- Lipets EN, Ataullakhanov FI. Global assays of hemostasis in the diagnostics of hypercoagulation and evaluation of thrombosis risk. Thromb J 2015;13:4. [Crossref] [PubMed]
- Kim HK, Song KS, Lee KR, et al. Comparison of plasma D-dimer and thrombus precursor protein in patients with operable breast cancer as a potential predictor of lymph node metastasis. Blood Coagul Fibrinolysis 2004;15:9-13. [Crossref] [PubMed]
- Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer 2011;11:123-34. [Crossref] [PubMed]
- Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell 2006;10:355-62. [Crossref] [PubMed]
- Gil-Bernabé AM, Lucotti S, Muschel RJ. Coagulation and metastasis: what does the experimental literature tell us? Br J Haematol 2013;162:433-41. [Crossref] [PubMed]
- Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012;119:933-9. [Crossref] [PubMed]
- Choi J, Ghoz HM, Peeraphatdit T, et al. Aspirin use and the risk of cholangiocarcinoma. Hepatology 2016;64:785-96. [Crossref] [PubMed]
- Cao Y, Nishihara R, Wu K, et al. Population-wide Impact of Long-term Use of Aspirin and the Risk for Cancer. JAMA Oncol 2016;2:762-9. [Crossref] [PubMed]
- Liang YL. Hypercoagulable state of patients after elective abdominal or pelvic surgery. Hebei Medical University, 2003.
- Man YN, Wang YN, Hao J, et al. Pretreatment plasma D-dimer, fibrinogen, and platelet levels significantly impact prognosis in patients with epithelial ovarian cancer independently of venous thromboembolism. Int J Gynecol Cancer 2015;25:24-32. [Crossref] [PubMed]
- Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. New Engl J Med 2012;366:610-8. [Crossref] [PubMed]
- Mi SC. Influence of surgical procedure improvement on complications and traditional Chinese medicine treatment in patients with lung cancer after argon-helium knife. Beijing University of Chinese Medicine, 2012.
- Han XD, Zhang X, Liu C, et al. Clinical investigation of hypercoagulable state of patients with non-small cell lung cancer. Progress in Modern Biomedicine 2015;5:92-6.
- Yang ZY, Zhao XH. Adverse events of 30 malignant cancer patients treated with huisheng oral solution. Chinese Patent Drug 1998;20:26.
- Fu J. pharmacological action and clinical application of huisheng oral solution. Shandong Medical and Pharmaceutical Journal 2011;51:110-1.
- Xu XH, Su J, Fu XY, et al. effect of huishengkoufuye on treatment and blood coagulation state in patients with mid-advanced non-small cell lung cancer after chemotherapy. Cancer Research on Prevention and Treatment 2011;38:695-700.
- Li YY, Luo DX, Chen H, et al. effect of huisheng oral solution on blood coagulation state of non-small cell lung cancer patients. Acta Academiae Medicinae Militaris Tertiae 2012;34:2259-63.