Pneumocystis jiroveci pneumonia in patients with non-Hodgkin’s lymphoma after Rituximab-containing regimen: two cases of report and literature review
Introduction
Pneumocystis jiroveci pneumonia (PCP), an opportunistic infection, is one of the major infectious complications in patients with human immunosuppressive virus (HIV) or in non-HIV immunocompromised patients subjected to chemotherapy. PCP is usually easy to be diagnosed but sometimes apt to be under-diagnosed for its atypical symptoms and signs. The occurrence of PCP is mainly due to the decrease of white blood cells especially CD4+ lymphocytes which influence the anti-infection ability of patient (1). Patients with low CD4+ lymphocyte counts usually have a higher risk of developing PCP (2).
Rituximab is a chimeric monoclonal antibody which targets the B cell-specific antigen CD20. Rituximab can reduce the number of B cells and has been increasingly used and proved rather effective in combination with other chemo-drugs in the treatment of non-Hodgkin lymphoma. However, as B cells play a vital role in generation of CD4+ T cells for defending PCP infection in the lungs, reduction of B cells could lead to insufficient generation of CD4+ T cells and subsequently higher risk of pneumocystis jiroveci infection. PCP was not reported in large scale, multi-centered clinical trials of Rituximab involved over 3,000 patients, and infrequently reported in patients with lymphoma who were treated with Rituximab-containing therapy in recent years.
Herein, we present two cases of patients with non-Hodgkin lymphoma who developed PCP after Rituximab-containing chemotherapy. PCP was confirmed by Giemsa-staining and both patients were treated successfully, with oral trimethoprim-sulfamethoxazole (TMP-SMZ) and corticosteroid therapy in one case and Caspofungin alone in the other respectively. We also reviewed the literature to give an overview including cause, diagnosis, treatment, and prophylaxis for PCP in patients with non-Hodgkin lymphoma who received Rituximab-containing regimen.
Case reports
Case 1
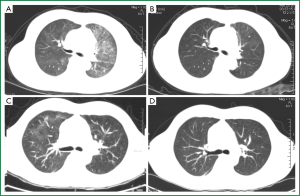
A 46-year-old female was diagnosed with large-B-cell lymphoma, grade IIb, in December 2011 by pathological observations from left supraclavicular lymph node biopsy specimen. On Dec 15th, she was given chemotherapy with Rituximab-CHOP regimen, including cyclophosphamide 750 mg/m2, day 1; doxorubicin 50 mg/m2, day 1; vincristine 2 mg, day 1; prednisolone 100 mg/day for 5 days. Rituximab was given at a dose of 600 mg, day 1. The patient well tolerated the treatment and discharged on Dec 20th with a normal blood white cell count (WBC 8.95×109/L), normal hepatic and renal functions. The chemotherapy was given in three cycles biweekly. Ten days after discharging, the patient presented with bone marrow suppression as peripheral blood WBC lowered to 1.59×109/L. GSM-GF injection was given for continually three days and the WBC counts rose to 21.9×109/L at day 3. On Jan 22nd, the patient presented with chest-tightness and dyspnea but no cough and fever. Chest CT scanning was performed and revealed obvious diffuse ground glass opacities (GGO) with interstitial thickening in both upper lungs (Figure 1A). A diagnostic bronchial-alveolar lavage (BAL) was immediately performed and bronchial-alveolar lavage fluid was collected. Under microscope foamy masses that highly suggestive of pneumocystis jiroveci were observed and the diagnosis was finally established by Giamsa-staining. The patient was given oral TMP-SMZ (400/80 mg, at every eight hours) and corticosteroid (30 mg, daily) therapy. A chest CT scanning three weeks after the TMP-SMX therapy demonstrated a complete disappearance of pulmonary infiltrates (Figure 1B).
Case 2
A 46-years old male was admitted to our hospital for a mass on left jaw. After operation, pathological examination revealed a diffuse large-B cell lymphoma in salivary gland. Immuno-labeling showed that CD20, CD79alpha, CD21, Ki-67, and bcl-2, 6 were all positive. The patient was wholly accessed and diagnosed as diffuse large-B-cell lymphoma, stage II A, IPI score 0, and given R-CHOP regimen (Rituximab 0.6, day 1, CTX 1.2, day 1, doxorubicin 100 mg, VCR 2 mg day 1, prednisone 100 mg, day 1-5). The patient well tolerated the chemotherapy and discharged on Dec 31st, 2011. The chemotherapy was repeated biweekly for 5 cycles. In March, 2012, he presented with cough, slight chest-tightness, and a fever of 37.5 degrees centigrade. He was admitted to hospital on April 2nd, 2012. Physical examination did not find any abnormal signs of the lung like rale. Peripheral blood cell count was normal (WBC 8.1×109/L, Neutrophilecyte 64%). A chest CT scanning was performed and showed ground glass opacities and enhanced pulmonary infiltrates in right upper lobe of the lung which suggested lung infection (Figure 1C). He was given levofloxacin and cefoperaxone. However the symptoms were not satisfactorily relieved. For the relatively typical manifestations on CT scanning film, special pathogens were suspected and a diagnostic bronchial alveolar lavage was maneuvered. Smear of BAL fluid revealed foamy masses highly suggestive of Pneumocystis jiroveci and Giamsa-staining was further performed. PCP was confirmed by typical morphological features of Pneumocystis jiroveci under microscope and the diagnosis was finally established. Due to a history of allergic to sulfa drugs, he was given Caspofungin (50 mg daily with 70 mg at day 1) instead of TMP-SMZ beginning on April 7th. The symptoms were relieved remarkably within a week. A Chest CT scanning was performed on April 12th, and demonstrated that most of the patches were absorbed and the pulmonary infiltrations were reduced (Figure 1D). For the satisfactory result of anti-Pneumocystis jiroveci treatment and no contradiction of chemotherapy, the patient accepted further R-CHOP chemotherapies. The patient was given 7 cycles of R-CHOP regimen all together. PCP was not found during the later chemotherapies.
Discussion
Rituximab, a chimeric human/mouse monoclonal antibody, can bind to the CD20 antigen presented on the surface of B lymphocytes, suppress the growth of B-cells and even kill them. Rituximab has been widely used in treatment of B-cell neoplasm especially the CD20-positive non-Hodgkin lymphoma (NHL) and remarkably enhanced the efficacy of chemotherapy. It has also been applied in the treatment of some other disorders and situations as Wegener’s Granulomatosis (3) systemic lupus erythematosus (SLE) (4), hematopoietic stem cell transplantation (5), rheumatoid arthritis (6), refractory nephrotic syndrome (7), and humoral renal transplant rejection (8).
With the increasing application of Rituximab, adverse events induced by Rituximab were documented continually. Among them, the reactivation of hepatitis B virus was the most common event. Secondly, PCP was reported in patients treated with Rituximab or Rituximab-including regimen (9). In 2007, Ame Kolstad and his colleagues reported six cases of PCP in B-cell lymphoma patients treated with the Rituximab-CHOEP-14 regimen. They concluded that depletion of B cells caused by Rituximab monotherapy did not increase the risk of PCP infections. When used in combination with other agents like steroids, Rituximab can increase the opportunity of PCP infection since it can impair the cellular immune system (10). Since then, reports of PCP in patients with lymphoma subjected to Rituximab continually emerged (11). The incidences of PCP in patients with lymphoma treated with Rituximab-containing chemotherapy were different in every report (Table 1). Ennishi D. et al. (2008) reported that 13 of 90 (14%) patients developed interstitial pneumonia (IP) during R-CHOP therapy, compared with none of 105 patients treated with CHOP alone as a historical control. In five IP patients, two were confirmed positive for Pneumocystis jirovecii (17). Venhuizen A. C. et al. [2008] found three cases of PCP in thirty patients during first-line treatment with Rituximab in combination with CHOP-14 for aggressive B-cell non-Hodgkin’s lymphoma (16). Katsuya H. et al. indicated 1 of 59 (1.7%) patients who received CHOP alone and 8 of 129 (6.2%) patients who were treated with R-CHOP experienced IP. Among the 8 patients who were diagnosed as IP during R-CHOP, three were confirmed having PCP (15). Kamel S. et al. even reported that among 47 consecutive patients treated with R-CHOP-14, five (11%) developed microbiologically proven PCP, with a further two (4%) having classical clinical and radiological features of PCP even though without microbiological confirmation (14).
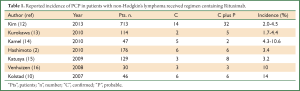
Full Table
The cause of increased incidence of PCP in those patients remains controversial. As corticosteroid was usually administrated in the same time, it is hard to conclude that the adverse events were induced by Rituximab alone. A history of Rituximab treatment might not be related to PCP (2). For the B-cell suppressive effect of Rituximab and B-cells play a pivot rule in the activation of CD4 T cells, the addition of Rituximab to chemotherapy was identified as an independent risk factor for IP (18). It is reasonable to deduce that PCP was associated with the use of Rituximab at least in part.
The number of white blood cells seems to be an important risk factor in the occurrence of PCP. Few researchers reported that CD4+ lymphocyte counts were normal in PCP patients (3). But most others demonstrated the opposite (11,12,19). For example, patients with CD4+ lymphocyte counts </=200/mm3 before chemotherapy had a higher risk of developing PCP (2). Among the patients treated by R-CHOP, 3 of 32 (9%) patients whose lymphocyte counts were <1,000/microL before chemotherapy developed PCP, while 70 patients whose lymphocyte counts were >1,000/microL did not (15). In a retrospective study including 529 patients, PCP was diagnosed in six patients whose absolute lymphocyte count was less than 1×109/L at diagnosis (18) and the reporter concluded that low lymphocyte count was an independent risk factor for PCP. Because of this conclusion, prophylaxis was highly recommended for patients with low CD4+ cell counts (10). In our report, both patients developed PCP during normal lymphocyte count period, which did not support that conclusion. But, one patient had a history of neutropenic period before the diagnosis of PCP. It is obvious that to estimate how long had the patient been infected by pneumocystis jiroveci before the diagnosis of PCP is difficult. Therefore, more data are needed to come to a more convincing conclusion.
The use of corticosteroid was also considered a risk factor for PCP (14,20,21). In a large consecutive series, systemic corticosteroid therapy, even in moderate doses, was administered to most patients during the month before the onset of PCP (22). The continuous use of steroids for conditions other than lymphoma could also significantly increase the risk of pulmonary infection including PCP (23).
The diagnosis of PCP is usually based on the symptoms, manifestations of chest-X-ray or CT scanning, and laboratory examinations. Most patients have fever, cough and dispnea. But it is not always the case. Some patients were asymptomatic (7,24). The symptoms can be atypical either. For example, case one in our report did not have cough and fever at all except dyspnea. On chest film, typical ground-glass opacities (GGO) can often be found. But these manifestations were not exclusive and often confused with other lung infection like viral pneumonia. In some cases, single or even multiple persistent granulomatous pulmonary nodules were presented (19,24). In laboratory technology, there are many methods including Giemsa-stain, Gomoris Methenamine silver nitrate stain, and PCR that are being used to confirm the existence of pneumocystis jiroveci (7,10,17). Up to date, the most sensitive method remains to be Giemsa-stain in which pneumocystis jiroveci appears to be “ping-pong”-like pink coccus under microscope. In our report, both cases were confirmed by Giemsa-staining.
The treatment and prophylaxis of PCP are relatively not difficult. In most reports, patients were treated successfully with TMP/SMX (11,13,17,25). In our report, case one was also successfully treated with TMP/SMX. TMP/SMX was also effective in preventing patient from suffering from PCP. In one study, none of patients who had SMZ developed PCP, while 6 of 176 (3.4%) patients who had no prophylaxis developed PCP (2). A 91% reduction in the occurrence of PCP was observed in another study (26). In another study, none of 121 patients who received PCP prophylaxis with TMP/SMX during chemotherapy developed PCP, while among 176 patients who had no prophylaxis, six (3.4%) developed PCP after starting chemotherapy, which demonstrated that TMP/SMX was effective for prophylaxis (2). Prophylaxis with TMP/SMX was found to be highly efficient and recommended for patients receiving the R-CHOP-14 regimen (25).
Caspofungin (brand name Cancidas) is a lipopeptide antifungal drug which is a member of a new class of antifungals termed the echinocandins. It can inhibit the enzyme β (1,3)-D-Glucan synthase and thereby disturb the integrity of the fungal cell wall. Laboratory research showed that Caspofungin had anti-pneumocystis activity (27). Caspofungin has been used with TMP/SMX for treatment of PCP (28,29), and recommended as a salvage treatment in PCP (30). The patient in case two in our report was treated with Caspofungin but not combined with TMP/SMX because he was oversensitive to TMP/SMX. He was also cured successfully, which suggested that Caspofungin alone could be also effective for treatment of PCP.
In conclusion, based on the literatures and data of cases we reported above, PCP is an opportunistic infection that can occur in patients with lymphoma treated with Rituximab-included regimen. On CT scanning film, granulomatous pulmonary nodules can also been seen instead of the typical ground grass opacities. TMP/SMX is effective in treatment and prophylaxis in most cases, and Caspofungin could be an effective alternative.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lund FE, Hollifield M, Schuer K, et al. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J Immunol 2006;176:6147-54. [PubMed]
- Hashimoto K, Kobayashi Y, Asakura Y, et al. Pneumocystis jiroveci pneumonia in relation to CD4+ lymphocyte count in patients with B-cell non-Hodgkin lymphoma treated with chemotherapy. Leuk Lymphoma 2010;51:1816-21. [PubMed]
- Hugle B, Solomon M, Harvey E, et al. Pneumocystis jiroveci pneumonia following rituximab treatment in Wegener’s granulomatosis. Arthritis Care Res (Hoboken) 2010;62:1661-4. [PubMed]
- Tsai MJ, Chou CW, Lin FC, et al. Pneumocystis jiroveci pneumonia in patients with systemic lupus erythematosus after rituximab therapy. Lupus 2012;21:914-8. [PubMed]
- Kato H, Yamamoto K, Taji H, et al. Interstitial pneumonia after autologous hematopoietic stem cell transplantation in B-cell non-hodgkin lymphoma. Clin Lymphoma Myeloma Leuk 2011;11:483-9. [PubMed]
- Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004;350:2572-81. [PubMed]
- Sato M, Ito S, Ogura M, et al. Atypical Pneumocystis jiroveci pneumonia with multiple nodular granulomas after rituximab for refractory nephrotic syndrome. Pediatr Nephrol 2013;28:145-9. [PubMed]
- Shelton E, Yong M, Cohney S. Late onset Pneumocystis pneumonia in patients receiving rituximab for humoral renal transplant rejection. Nephrology (Carlton) 2009;14:696-9. [PubMed]
- Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol 2010;47:187-98. [PubMed]
- Kolstad A, Holte H, Fosså A, et al. Pneumocystis jirovecii pneumonia in B-cell lymphoma patients treated with the rituximab-CHOEP-14 regimen. Haematologica 2007;92:139-40. [PubMed]
- Chang H, Yeh HC, Su YC, et al. Pneumocystis jiroveci pneumonia in patients with non-Hodgkin’s lymphoma receiving chemotherapy containing rituximab. J Chin Med Assoc 2008;71:579-82. [PubMed]
- Kim T, Choi SH, Kim SH, et al. Point prevalence of Pneumocystis pneumonia in patients with non-Hodgkin lymphoma according to the number of cycles of R-CHOP chemotherapy. Ann Hematol 2013;92:231-8. [PubMed]
- Kurokawa T, Kaya H, Yoshida T. Two cases of Pneumocystis jiroveci pneumonia with non-Hodgkin’s lymphoma after CHOP-based chemotherapy containing rituximab. J Clin Exp Hematop 2010;50:159-62. [PubMed]
- Kamel S, O’Connor S, Lee N, et al. High incidence of Pneumocystis jirovecii pneumonia in patients receiving biweekly rituximab and cyclophosphamide, adriamycin, vincristine, and prednisone. Leuk Lymphoma 2010;51:797-801. [PubMed]
- Katsuya H, Suzumiya J, Sasaki H, et al. Addition of rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy has a high risk of developing interstitial pneumonia in patients with non-Hodgkin lymphoma. Leuk Lymphoma 2009;50:1818-23. [PubMed]
- Venhuizen AC, Hustinx WN, van Houte AJ, et al. Three cases of Pneumocystis jirovecii pneumonia (PCP) during first-line treatment with rituximab in combination with CHOP-14 for aggressive B-cell non-Hodgkin’s lymphoma. Eur J Haematol 2008;80:275-6. [PubMed]
- Ennishi D, Terui Y, Yokoyama M, et al. Increased incidence of interstitial pneumonia by CHOP combined with rituximab. Int J Hematol 2008;87:393-7. [PubMed]
- Huang YC, Liu CJ, Liu CY, et al. Low absolute lymphocyte count and addition of rituximab confer high risk for interstitial pneumonia in patients with diffuse large B-cell lymphoma. Ann Hematol 2011;90:1145-51. [PubMed]
- Chang H, Shih LY, Wang CW, et al. Granulomatous Pneumocystis jiroveci pneumonia in a patient with diffuse large B-cell lymphoma: case report and review of the literature. Acta Haematol 2010;123:30-3. [PubMed]
- Sepkowitz KA, Brown AE, Telzak EE, et al. Pneumocystis carinii pneumonia among patients without AIDS at a cancer hospital. JAMA 1992;267:832-7. [PubMed]
- Worth LJ, Dooley MJ, Seymour JF, et al. An analysis of the utilisation of chemoprophylaxis against Pneumocystis jirovecii pneumonia in patients with malignancy receiving corticosteroid therapy at a cancer hospital. Br J Cancer 2005;92:867-72. [PubMed]
- Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc 1996;71:5-13. [PubMed]
- Lim KH, Yoon HI, Kang YA, et al. Severe pulmonary adverse effects in lymphoma patients treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen plus rituximab. Korean J Intern Med 2010;25:86-92. [PubMed]
- Kumar N, Bazari F, Rhodes A, et al. Chronic Pneumocystis jiroveci presenting as asymptomatic granulomatous pulmonary nodules in lymphoma. J Infect 2011;62:484-6. [PubMed]
- Hardak E, Oren I, Dann EJ, et al. The increased risk for pneumocystis pneumonia in patients receiving rituximab-CHOP-14 can be prevented by the administration of trimethoprim/sulfamethoxazole: a single-center experience. Acta Haematol 2012;127:110-4. [PubMed]
- Green H, Paul M, Vidal L, et al. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc 2007;82:1052-9. [PubMed]
- Cushion MT, Collins MS. Susceptibility of Pneumocystis to echinocandins in suspension and biofilm cultures. Antimicrob Agents Chemother 2011;55:4513-8. [PubMed]
- Beltz K, Kramm CM, Laws HJ, et al. Combined trimethoprim and caspofungin treatment for severe Pneumocystis jiroveci pneumonia in a five year old boy with acute lymphoblastic leukemia. Klin Padiatr 2006;218:177-9. [PubMed]
- Zhang JC, Dai JY, Fan J, et al. The treatment of pneumocystis Carinii pneumonia with caspofungin in elderly patients: a case report and literature review. Zhonghua Jie He He Hu Xi Za Zhi 2006;29:463-5. [PubMed]
- Armstrong-James D, Stebbing J, John L, et al. A trial of caspofungin salvage treatment in PCP pneumonia. Thorax 2011;66:537-8. [PubMed]


