Morphine in acute heart failure
Acute pulmonary oedema is a common condition in the emergency room, associated with considerable mortality. Morphine has since a long time, been used in patients with acute pulmonary oedema due to its anticipated anxiolytic and vasodilatory properties; however a discussion about the benefits and risks has been raised recently (1). There are no large randomised controlled trials supporting the use of morphine in the treatment of patients with acute heart failure (AHF). A certain vasodilation has been described after morphine administration (2), but the evidence for this mechanism is relatively poor and morphine-induced anxiolysis may possibly be the most important factor of morphine in pulmonary oedema and therefore some authors have suggested benzodiazepines as an alternative treatment (3).
Therefore, the European Society of Cardiology (ESC) guidelines on heart failure (4) state that routine use of opiates is not recommended and they may only be cautiously considered in patients with severe dyspnoea, mostly with pulmonary oedema. The American Heart Association (AHA)/American College of Cardiology (ACC) only support the use of morphine for palliative care in end-stage heart failure (5).
In a recent study published in Chest (6), the authors included 6,516 consecutive AHF patients presenting to 34 Spanish emergency departments from 2011 to 2014. The subjects were divided into those with or without intravenous morphine treatment. The primary outcome was 30-day all-cause mortality, and secondary outcomes were mortality at different intermediate time points, in-hospital mortality, and length of hospital stay. To adjust for basal differences between the groups, a propensity-score in order to match the group treated with morphine with the group without intravenous morphine treatment was used to adjust for 46 different epidemiological, baselines, clinical and therapeutic factors. After 30 days the mortality was 26.7% in those treated with morphine compared to 8.6% mortality in those without acute morphine treatment. After propensity score matching, with 275 paired patients in each group, patients treated with morphine had significantly higher 30-day mortality (20.0% vs. 12.7%) in those not treated with morphine. Mortality was increased at every intermediate time point, although the greatest risk was at the shortest time (3 days). The authors concluded that the propensity score-matched analysis suggested that the use of intravenous morphine in AHF could be associated with increased 30-day mortality.
However, this was not a randomized clinical trial. In the clinic situation, morphine is more likely to be given to the sickest patients and that was obviously the case in this study as well. Patients given morphine had higher rates of ischaemic heart disease, cerebrovascular disease, peripheral artery disease and dementia and had a worse functional status, with a higher New York Heart Association class (NYHA III–IV). In addition patients in the morphine group were more likely to receive intravenous nitrates, vasoactive drugs, non-invasive ventilation and any kind of ventilatory support. In the entire cohort, 635 (9.7%) patients died within 30 days after ED attendance: 26.7% vs. 8.6%, respectively, in the morphine and non-morphine groups (P<0.001). With the aim to adjust for these basal differences between the two groups the authors created a propensity score-matched analysis using the 24 significant baseline variables that differed between the two groups. Thus, the matching provided 275 balanced paired cases. Indeed, residual confounding can never be fully excluded, despite that the authors used propensity score matching to minimize this risk. Furthermore, the quality of data might not be as high as in a properly monitored randomized clinical trial. Thus, the results should be verified in randomized clinical trials.
The results are in line with most previous studies. Previously, Peacock and colleagues published data from the large (more than 147,000 patients) ADHERE registry (7). Almost 21,000 patients received morphine and after adjusting for factors known to be associated with increased hospital mortality, including advanced age, troponin elevation and hypertension; morphine remained an independent predictor of mortality. In addition, significant correlations between morphine administration and increased intubation rate, more intensive care unit admissions and prolonged hospitalisation were found. In a similar but smaller study from 2003 to 2007 in UK, no significant correlation between morphine administration and mortality was observed (8). However, there was neither any correlation between the use of morphine and improvement in respiratory distress, measured in patient perceived breathlessness over the first hour. In a retrospective study in Israel containing 2,336 patients, morphine administration was associated with an increased mortality (9). However, after propensity score matching this association became non-significant. Both in the European and the Israeli study, there was a tendency to aggravation associated with morphine treatment, but the results were not significant. None of the studies tended to demonstrate an improved prognosis when morphine was given.
Why morphine in AHF may cause harm
It is described that between one fifth and one third of patients experience nausea when using opioids (10,11). Nausea and vomiting is also very unfortunate because of the risk for aspiration, if there is a need for treatment with continuous positive pressure (CPAP). Furthermore, treatment with morphine may cause an attenuated platelet inhibition after per oral treatment with these drugs. Kubica and co-workers have present findings of attenuated platelet inhibition by ticagrelor induced by the concomitant administration of morphine (12). The authors tested a 5 mg intravenous dose of morphine against placebo in 70 patients with acute myocardial infarction (AMI), followed by a 180 mg loading dose of ticagrelor. The measured parameters included ticagrelor plasma concentrations and several platelet function tests. All assessments showed a delayed ticagrelor plasma appearance as well as a weaker antiplatelet effect in the morphine group. Hobl and co-workers found a decreased clopidogrel response in healthy volunteers who received morphine (13). Further, Parodi et al. found that both prasugrel and ticagrelor displayed a delayed activity when given concomitantly with morphine in patients with STEMI undergoing primary percutaneous coronary intervention (PCI) (14). Thus, in patients with AMI and AHF morphine may delay the antiplatelet effect which may be harmful.
It has also been found that morphine induces depression of the myocardium that results in decreased heart rate and cardiac output (15).
Morphine may also cause respiratory depression (16) which possibly may lead to intubation and ventilator treatment.
Morphine is still used for pulmonary oedema in spite of poor scientific background data. A randomised, controlled study is necessary in order to determine the effect—and especially the risk—when using morphine for pulmonary oedema. Since the positive effects are not sufficiently documented, and since the risk for increased mortality cannot be ruled out, one can advocate that the use should be avoided.
How to treat AHF in evidenced based medicine practice
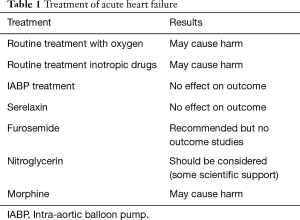
Thus, the treatment of AHF has not developed much since the 1960s (Table 1). Rather, our treatment options may have decreased. Oxygen should not be used routinely in non-hypoxaemic patients, as it causes vasoconstriction and a reduction in cardiac output. Supplemental oxygen therapy in patients with ST-elevation-myocardial infarction but without hypoxia may increase early myocardial injury and was associated with larger myocardial infarct size assessed at 6 months (17).
Full table
Inotropic agents are not recommended unless the patient is symptomatically hypotensive or hypoperfused because of safety concern (4,18,19). Intra-aortic balloon pump (IABP) is not routinely recommended in cardiogenic shock (4,20,21). Despite, promising data from small studies (22), Serelaxin failed to meet the primary endpoints of the phase 3 RELAX-AHF-2 trial, according to late breaking results presented at Heart Failure 2017 and the 4th World Congress on AHF.
Treatment with furosemide, which inhibits reabsorption of sodium in Henle’s loop and distal tubule and thereby increasing excretion of fluids through the kidney, has a class IC recommendation in the ESC GLs on heart failure. Thus, again we lack scientific evidence on the effect. Then there is nitroglycerin, which via cGMP and smooth muscle relaxation induces vasodilatation. In AHF the ESC GLs has a class IIa B recommendation, thus this treatment should be considered and there are some evidence supporting this action.
In conclusion, our treatment of AHF (in contrast to the treatment of chronic heart failure) has not developed much during the last 50 years. Routine treatment with oxygen and inotropic drugs may be harmful, and IABP treatment has not shown effect on outcome. Serelaxin failed to meet the primary endpoints in a large phase 3 study. Now we may add morphine treatment on this failure list. Treatment with furosemide and nitroglycerin remains on the list but also here the underlying scientific evidence is poor.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Ellingsrud C, Agewall S. Morphine in the treatment of acute pulmonary oedema--Why? Int J Cardiol 2016;202:870-3. [Crossref] [PubMed]
- Vismara LA, Leaman DM, Zelis R. The effects of morphine on venous tone in patients with acute pulmonary edema. Circulation 1976;54:335-7. [Crossref] [PubMed]
- Mattu A, Martinez JP, Kelly BS. Modern management of cardiogenic pulmonary edema. Emerg Med Clin North Am 2005;23:1105-25. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2016;68:1476-88. [Crossref] [PubMed]
- Miró Ò, Gil V, Martín-Sánchez FJ, et al. Morphine use in the emergency department and outcomes of patients with acute heart failure: A propensity score-matching analysis based on the EAHFE Registry. Chest 2017. [Epub ahead of print]. [Crossref]
- Peacock WF, Hollander JE, Diercks DB, et al. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J 2008;25:205-9. [Crossref] [PubMed]
- Gray A, Goodacre S, Seah M, et al. Diuretic, opiate and nitrate use in severe acidotic acute cardiogenic pulmonary oedema: analysis from the 3CPO trial. QJM 2010;103:573-81. [Crossref] [PubMed]
- Iakobishvili Z, Cohen E, Garty M, et al. Use of intravenous morphine for acute decompensated heart failure in patients with and without acute coronary syndromes. Acute Card Care 2011;13:76-80. [Crossref] [PubMed]
- Smith HS, Laufer A. Opioid induced nausea and vomiting. Eur J Pharmacol 2014;722:67-78. [Crossref] [PubMed]
- Cepeda MS, Farrar JT, Roa JH, et al. Ethnicity influences morphine pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2001;70:351-61. [Crossref] [PubMed]
- Kubica J, Adamski P, Ostrowska M, et al. Morphine delays and attenuates ticagrelor exposure and action in patients with myocardial infarction: the randomized, double-blind, placebo-controlled IMPRESSION trial. Eur Heart J 2016;37:245-52. [Crossref] [PubMed]
- Hobl EL, Stimpfl T, Ebner J, et al. Morphine decreases clopidogrel concentrations and effects: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol 2014;63:630-5. [Crossref] [PubMed]
- Parodi G, Bellandi B, Xanthopoulou I, et al. Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv 2014.8. [PubMed]
- Riggs TR, Yano Y, Vargish T. Morphine depression of myocardial function. Circ Shock 1986;19:31-8. [PubMed]
- Radke JB, Owen KP, Sutter ME, et al. The effects of opioids on the lung. Clin Rev Allergy Immunol 2014;46:54-64. [Crossref] [PubMed]
- Stub D, Smith K, Bernard S, et al. Air Versus Oxygen in ST-Segment-Elevation Myocardial Infarction. Circulation 2015;131:2143-50. [Crossref] [PubMed]
- Elkayam U, Tasissa G, Binanay C, et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J 2007;153:98-104. [Crossref] [PubMed]
- Belletti A, Castro ML, Silvetti S, et al. The Effect of inotropes and vasopressors on mortality: a meta-analysis of randomized clinical trials. Br J Anaesth 2015;115:656-75. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287-96. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet 2013;382:1638-45. [Crossref] [PubMed]
- Díez J, Ruilope LM. Serelaxin for the treatment of acute heart failure: a review with a focus on end-organ protection. Eur Heart J Cardiovasc Pharmacother 2016;2:119-30. [Crossref] [PubMed]


