Bronchoscopy with endobronchial ultrasound guided transbronchial needle aspiration vs. transthoracic needle aspiration in lung cancer diagnosis and staging
Introduction
Staging of lung cancer is an important step that dramatically affects therapy and prognosis. This is highly dependent upon tissue evaluation of the mediastinal and hilar nodal status to categorize risk and define treatment strategy. Patients with lower-stage disease (IA, IB, IIA, IIB) can undergo surgical resection with curative intent, whereas patients with clinical stage IIIA often receive neoadjuvant chemotherapy, radiotherapy, and surgery or definitive chemotherapy and radiotherapy, while IIIB and above typically receive palliative chemotherapy +/− radiation. As such, guidelines developed by organizations worldwide, including the American College of Chest Physicians (ACCP) and the National Institute of Clinical Excellence (NICE), recommend nodal staging of all patients whose initial workup shows concern for mediastinal involvement (1,2).
Each of the many available staging methods (e.g., mediastinoscopy, video-assisted thoracoscopic surgery (VATS), endobronchial ultrasound guided trans-bronchial needle aspiration (EBUS-TBNA), conventional transbronchial needle aspiration, and transthoracic needle aspiration (TTNA), has its associated diagnostic yield and risk of complications, and it is often the availability of resources and local expertise that guides the initial diagnostic approach. Unfortunately, some of these procedures cannot evaluate mediastinal nodes and thus are not guideline-consistent, but are still widely used at many institutions. In a recent review of Medicare databases, only 56% of patients had mediastinal sampling before treatment, and only 21% had guideline-consistent diagnostic evaluations (1-4).
Additionally, many patients may require multiple procedures to obtain adequate tissue for tumor subtyping and biomarker analysis as well as to define the clinical stage. This not only incurs higher cost and places patients at additional risk of complications but can be associated with delays in initiation of care.
Over the last several years, EBUS-TBNA has emerged as a minimally invasive procedure with an excellent diagnostic yield and in many centers has become the initial procedure of choice in the diagnosis and staging of patients with suspected lung cancer (5,6). Benefits of EBUS-TBNA include the ability to sample the majority of mediastinal and hilar nodes, as well as obtain adequate tissue for molecular marker analysis (7,8). As with any procedure, however, EBUS-TBNA is associated with its own learning curve and is not available at all centers (9). The effect of a dedicated EBUS program on safety and timeliness of staging evaluations has not been addressed. We sought to determine the safety and need for additional biopsies for patients staged with EBUS-TBNA compared to other biopsy techniques in two academic medical centers within the same healthcare system.
Methods
Study design
The study was approved by the Johns Hopkins University institutional review board (IRB #NA_00048528). We utilized a retrospective cohort design to analyze all patients undergoing lung/lymph node biopsies for any indication between January 1, 2013 and December 31, 2013. The analysis was performed at two academic medical centers within the same university system with a multidisciplinary thoracic oncology program, one of which had a dedicated EBUS program (site 1) and the other without such a program (site 2). In this study, a dedicated EBUS program was defined as performing >170 EBUS procedures per year, consistent with the designation ‘high-volume center’ used in a prior American College of Chest Physicians Quality Improvement Registry (10). Patients were included in each cohort if biopsies showed a diagnosis of non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC) on surgical pathology or cytopathology. Exclusion criteria included age <18 years or recurrence of a known prior NSCLC/SCLC.
Characteristics and end points
Patient characteristics included age, sex, forced expiratory volume over one second (FEV1), tobacco consumption (pack-years), tumor size & location, presence and size of adenopathy, TNM stage (based on the IASLC 7th Edition) (11), initial biopsy method, location of initial biopsy, tumor subtype, adequacy of molecular marker analysis, subsequent biopsy procedures, complications, and time to initiation of therapy. The primary endpoints were the rate of patients requiring a second biopsy procedure and the rate of complications from their biopsies. Need for a second biopsy was defined as those patients whose primary sample was non-diagnostic, contained insufficient tissue for tumor subtyping, or did not fully stage the patient per guidelines resulting in the need for an additional staging procedure. Complications were defined as uncomplicated pneumothorax (detected radiographically within 24 h of a procedure but not requiring tube thoracostomy), complicated pneumothorax (detected radiographically within 24 h after a procedure and requiring tube thoracostomy), hypoxemic respiratory failure (SpO2 <90% by pulse oximetry due to any etiology and requiring hospitalization), or death. Of note, surgical resections frequently required post-thoracotomy chest tubes, so pneumothorax was not counted in this subgroup.
Method of biopsy
In our series, EBUS-TBNA was performed by a staff of three interventional pulmonologists, TTNA was performed by interventional radiologists, and conventional bronchoscopic techniques (bronchoalveolar lavage, bronchoscopic brushing, and non-EBUS guided transbronchial biopsies) were performed by general pulmonologists, well trained in all standard bronchoscopic techniques (though not EBUS). Surgical biopsies were performed exclusively as VATS for simultaneous tumor resection with lymph-node dissection for low-stage tumors.
Statistical analysis
We summarized descriptive data as means ± CI or proportions as appropriate. Unadjusted analyses were performed using t-test or chi-squared testing. Variables that were significant on univariate analyses or which were felt to be potentially clinically relevant were included in multivariate logistic regression models to predict the need for a second procedure or complications. These variables included demographic features age, sex, and tumor location. A P value <0.05 was considered statistically significant. We used STATA version 13 (STATA Corp, College Station, TX, USA) for all analyses.
Results
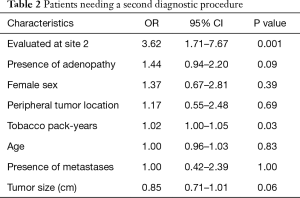
Eight hundred and thirty adults underwent lung or lymph node biopsies during the study period. Of these, 285 had a new diagnosis of NSCLC/SCLC. The initial characteristics of these patients are described in Table 1. Pre-procedural CT and PET imaging were used to determine an initial TNM staging for biopsy planning according to 7th edition IASLC classification (11) and are described in Table 2. Adenocarcinoma represented the largest proportion of tumor subtypes.

Full table
Full table
In our multivariate regression model of patients who required a second biopsy, the factor most predictive of the need for a second biopsy was the medical center at which they were biopsied (OR 3.62, 95% CI: 1.71–7.67, P=0.001). Additional biopsies were also positively correlated with the patient’s tobacco pack-year burden (OR 1.02, 95% CI: 1.00–1.05, P=0.03) and negatively-correlated with increasing tumor size (OR 0.85, 95% CI: 0.71–1.01, P=0.06), suggesting heavy smokers and those with smaller tumors were more likely to have inconclusive initial biopsy results. This was not a feature of the diagnostic yield of TTNA (66% at site 1 and 73% at site 2, P=0.42). There were no significant differences in age, gender, or location of tumor, or the presence of adenopathy or metastases (Table 2).
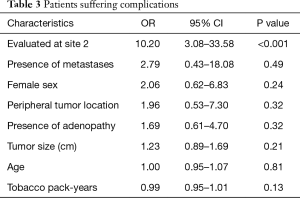
The only feature predictive of whether patients suffered complications was again the medical center at which they were biopsied, with there being a much higher risk of complications if the biopsy was done at site 2 (the non-EBUS hospital) (OR 10.2, 95% CI: 3.08–33.58, P<0.001) (Table 3). No other characteristics were associated with complications, including pack-year burden or tumor size.
Full table
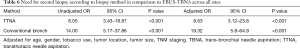
Given the apparent relationship between institutional site of biopsy and these outcomes (Table 4), we analyzed the procedural methods used at each institution. In addition to experiencing higher patient volumes, site 1 predominantly performed EBUS-TBNA and surgical biopsies as opposed to the conventional bronchoscopic and transthoracic techniques predominantly utilized at site 2 (Table 5).

Full table

Full table
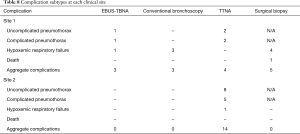
To control for these institutional preferences, we performed a second multivariate analysis comparing EBUS-TBNA to conventional bronchoscopy and TTNA across all sites. Conventional bronchoscopy and TTNA use, regardless of site, were associated with higher rates of complications and need for multiple biopsies (Tables 6,7). These complications are listed in Table 8.
Full table

Full table
Full table
Discussion
We report the largest comparison of patients undergoing evaluation for presumed lung cancer at two large multidisciplinary medical centers within the same university system. Our results show that patients who underwent initial evaluation with EBUS have fewer complications and less need for an additional procedure to perform proper staging or obtain adequate tissue for molecular marker analysis. In a secondary analysis, EBUS biopsies outperformed conventional bronchoscopy and TTNA-guided biopsies in these outcomes as well.
It is expected that higher-volume centers would have lower complication rates (3,12-14). Previously published data describe an overall lower rate of complications for EBUS-TBNA in comparison to TTNA or conventional transbronchial biopsy; pneumothorax rates have been reported at 0.5%, 4% and 15% for EBUS-TBNA, conventional transbronchial biopsy, and TTNA respectively (5,15,16). Nevertheless, as seen in our data and nationally, guideline-inconsistent use of higher risk procedures is still widespread, even at multidisciplinary institutions (1-4).
In this study, complications associated with EBUS-TBNA were far less severe than those seen with other methods of obtaining a tissue diagnosis. Among the three recorded complications after EBUS-TBNA, two were pneumothoraces (one requiring a chest tube), the other was a transient hypoxemic event requiring brief hospital admission. VATS procedures in this study had only five recorded complications (pneumothorax was not defined as a complication following thoracostomy). These included five episodes of hypoxemic respiratory failure (persistent bronchopleural fistula, hematoma requiring subsequent surgical drainage, subcutaneous emphysema requiring ICU admission, and two episodes of pneumonia requiring mechanical ventilation) one of which resulted in death. All 18 complications after TTNA represented pneumothoraces, 9 of which required chest tube placement including one which required operative extraction of a retained needle tip.
This is the first study to compare the need for additional procedures to adequately stage the patient or obtain enough tissue for histologic and molecular profiling. Our data indicate that patients who underwent EBUS at a dedicated EBUS program as their primary diagnostic method were less likely to require subsequent procedures. This finding is consistent with international data showing a favorable sensitivity and specificity profile EBUS-TBNA (1,17). As EBUS-TBNA has already been shown in to be more cost-effective than mediastinoscopy, conventional bronchoscopy, and transthoracic needle biopsy this is yet another reason that EBUS-TBNA should be the initial procedure of choice in evaluating patients with suspected lung cancer (18-21). As the diagnostic yield of TTNA was not significantly different between the sites, it is unlikely that the need for diagnosis, as opposed to staging, accounted for the requirement of a 2nd procedure. Clearly, not all patients require staging and a tissue diagnosis prior to surgical resection. The ACCP Lung Cancer guidelines suggests that resection and lymph node dissection can be considered for medically operable patients with tumors <2 cm (cT1a) and no CT or FDG-PET evidence of mediastinal adenopathy (1).
Furthermore, the need for fewer biopsy procedures implies a shorter time to diagnosis and initiation of therapy, which may correlate with improved therapeutic outcomes. Although the retrospective nature of these data did not allow for evaluation of mortality, at least one other study has suggested that EBUS-TBNA use is associated with prolonged survival (22).
Limitations
This analysis was only performed within a single university system. While this helps to minimize differences in institutional resources, practice variation and demographics, it is not clear whether these results are generalizable to other multidisciplinary institutions or non-academic medical centers. Minor population differences were noted between each institution; pack-year burden was higher at site 2, which may partially explain a higher complication rate. That being said, peripheral tumors were more common at site 1 which may have decreased biopsy success and increased complication rates. FEV1 data, while not significantly different between institutional populations, was only available in 168 of our patients and a logistic regression including this variable in our endpoint analysis was not sufficiently powered for this study. Likewise, formal CT grading of emphysema severity was not performed. EBUS-TBNA was primarily performed by a small group of trained interventional pulmonologists, who have a high volume practice, which may give rise to a better profile of success and complication rates for EBUS-TBNA than might be seen at lower volume institutions (12,23). As ours is a teaching institution, it was impossible to retrospectively determine the degree to which pulmonary fellows, interventional pulmonary fellows, interventional radiology fellows and thoracic surgery fellows participated in the procedures and though this may have influenced procedural complications, it likely would not significantly influence diagnostic yield. The complication rate of TTNA was higher than the reported rates of 15–40%, and this may have been due to several factors including number of needle passes, degree of underlying emphysema and use of trainees during the procedure. Nonetheless, the numbers represent real-world experience at an academic institution. Finally, surgical biopsies at our institution were predominantly performed as VATS procedures by thoracic surgeons for predominantly T1a lesions. Our study was not powered to compare EBUS-TBNA to VATS procedures for this patient population.
Conclusions
Our study suggests that EBUS-TBNA should be the initial modality to obtain a diagnosis, appropriately stage patients and obtain necessary tissue for tumor markers in the evaluation of patients with suspected lung cancer. This approach resulted in fewer complications and reduced the need for additional procedures. In the changing landscape of diagnosing and staging lung cancer, evaluation by EBUS-TBNA is the safest and most efficient route to therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Johns Hopkins University institutional review board (IRB #NA_00048528).
References
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Baldwin DR, White B, Schmidt-Hansen M, et al. Diagnosis and treatment of lung cancer: summary of updated NICE guidance. BMJ 2011;342:d2110. [Crossref] [PubMed]
- Ost DE, Niu J, Elting LS, et al. Determinants of practice patterns and quality gaps in lung cancer staging and diagnosis. Chest 2014;145:1097-113. [Crossref] [PubMed]
- Ost DE, Jim Yeung SC, Tanoue LT, et al. Clinical and organizational factors in the initial evaluation of patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e121S-41S.
- Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest 2013;143:1044-53. [Crossref] [PubMed]
- Adams K, Shah PL, Edmonds L, et al. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757-62. [Crossref] [PubMed]
- Yarmus L, Akulian J, Gilbert C, et al. Optimizing endobronchial ultrasound for molecular analysis. How many passes are needed? Ann Am Thorac Soc 2013;10:636-43. [Crossref] [PubMed]
- Navani N, Brown JM, Nankivell M, et al. Suitability of Endobronchial Ultrasound-guided Transbronchial Needle Aspiration Specimens for Subtyping and Genotyping of Non-Small Cell Lung Cancer: A Multicenter Study of 774 Patients. Am J Respir Crit Care Med 2012;185:1316-22. [Crossref] [PubMed]
- Stather DR, Chee A, MacEachern P, et al. Endobronchial ultrasound learning curve in interventional pulmonary fellows. Respirology 2015;20:333-9. [Crossref] [PubMed]
- Ost DE, Ernst A, Lei X, et al. AQuIRE Bronchoscopy Registry Diagnostic yield of endobronchial ultrasound-guided transbronchial needle aspiration: results of the AQuIRE Bronchoscopy Registry. Chest 2011;140:1557-66. [Crossref] [PubMed]
- Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [Crossref] [PubMed]
- Folch E, Majid A. Point: are >50 supervised procedures required to develop competency in performing endobronchial ultrasound-guided transbronchial needle aspiration for mediastinal staging? Yes. Chest 2013;143:888-91. [Crossref] [PubMed]
- Eggeling S, Martin T, Böttger J, et al. Invasive staging of non-small cell lung cancer--a prospective study. Eur J Cardiothorac Surg 2002;22:679-84. [Crossref] [PubMed]
- Loscertales J, Quero Valen Zuela F, Congregado M, et al. Video-assisted thoracic surgery lobectomy: results in lung cancer. J Thorac Dis 2010;2:29-35. [PubMed]
- Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 2011;155:137-44. [Crossref] [PubMed]
- Pue CA, Pacht ER. Complications of fiberoptic bronchoscopy at a university hospital. Chest 1995;107:430-2. [Crossref] [PubMed]
- Schmid-Bindert G, Wang Y, Jiang H, et al. EBUS-TBNA provides highest RNA yield for multiple biomarker testing from routinely obtained small biopsies in non-small cell lung cancer patients - a comparative study of three different minimal invasive sampling methods. PLoS One 2013;8:e77948. [Crossref] [PubMed]
- Sharples LD, Jackson C, Wheaton E, et al. Clinical effectiveness and cost-effectiveness of endobronchial and endoscopic ultrasound relative to surgical staging in potentially resectable lung cancer: results from the ASTER randomised controlled trial. Health Technol Assess 2012;16:1-75. iii-iv. [Crossref] [PubMed]
- Kunst PW, Eberhardt R, Herth FJ. Combined EBUS Real Time TBNA and Conventional TBNA are the Most Cost-effective Means of Lymph Node Staging. J Bronchology 2008;15:17-20. [Crossref]
- Lee KA, Raval AA, Amir L. Cost-effectiveness of endobronchial percutaneous biopsy compared with transthoracic biopsy for diagnosis of peripheral lung lesions. Lung Cancer Manag 2014;3:135-48. [Crossref]
- Tan S, Sharma K, Tham KY, et al. Comparing performance and cost of EBUS-TBNA versus other methods for diagnosis and staging of non-small cell lung cancer (NSCLC). Eur Respir J 2014;44:340.
- Navani N, Nankivell M, Lawrence DR, et al. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. Lancet Respir Med 2015;3:282-9. [Crossref] [PubMed]
- Feller-Kopman DJ, Brigham E, Lechtzin N, et al. Training perspective: the impact of starting an endobronchial ultrasound program at a major academic center on fellows training of transbronchial needle aspiration. Ann Am Thorac Soc 2013;10:127-30. [Crossref] [PubMed]


