Video-assisted thoracoscopic surgery for intrathoracic first rib resection in thoracic outlet syndrome
Introduction
Thoracic outlet syndrome (TOS) is a disease in which weakness, swelling, a tingling sensation, and/or pain of the upper extremities develop in a specific posture due to impingement of the brachial plexus and the subclavian vasculature, the latter of which is located between the anterior scalene muscle, first rib, and middle scalene muscle (1,2). Although the first rib is rarely fractured by blunt trauma, it can cause adjacent neurovascular compression if this occurs (3).
Non-operative interventions, including botulinum toxin injection into the scalene muscles and physical therapy, can be applied to relieve the symptoms of TOS (4). However, first rib resection is helpful for releasing the impingement of the subclavian vasculature and the brachial plexus in the thoracic outlet when symptoms remain intractable after these interventions (5,6).
Historically, the first rib has been removed through extrathoracic approaches, including posterior, supraclavicular, and transaxillary (1,7-10). Although many studies have reported extrathoracic first rib resection, few reports of intrathoracic approaches have been described. In this study, we present video-assisted thoracoscopic surgery for intrathoracic first rib resection (VATS-IFRR) in patients with TOS.
Methods
Patients
Eight patients underwent VATS-IFRR between 2009 and 2014. The symptoms, causes, operative times, complications, chest tube indwelling times, durations of hospital stay after operation, pain scores, symptom improvement rates, and symptom recurrence rates of the patients were investigated. This study was approved by the Ethic Committee on Clinical Investigation for Human Research (IRB) at Korea University Ansan Hospital (Ansan, South Korea) (No. AS170309-002). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Surgical procedure
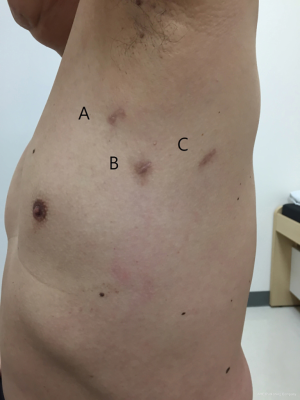
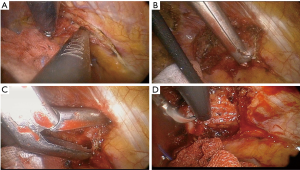
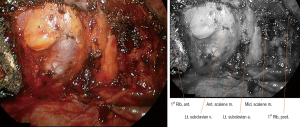
VATS-IFRR was performed through three ports in the following locations: anterior axillary line (3rd intercostal space, 5 mm), mid axillary line (5th intercostal space, 11.5 mm), and posterior axillary line (6th intercostal space, 5 mm) (Figure 1, Patient 8). The surgical space was obtained under one-lung selective ventilation and the use of a 5-mm, rigid 30° thoracoscope. After exact identification of the first rib, the parietal pleura of the first rib was opened using a 5-mm endoscopic grasper and either a hook-type electrocautery probe or an ultrasonic shear probe (Figures 2,3, Patient 7). The anterior margin of the resected first rib was the far anterior part where the subclavian vein runs across the rib, whereas the posterior margin was near the costovertebral junction of the rib and the far posterior part where the subclavian artery runs across the rib. The rib was cut using a 4.5-mm width Kerrison punch and a long peapod intervertebral disc rongeur (Figures 2,4, Patient 7). The first rib was then separated from the subclavian vein, anterior scalene muscle, subclavian artery, and middle scalene muscle using endoscopic graspers and a hook-type electrocautery probe (Figures 2,5, Patient 7). After removal of the first rib, thoracic outlet structures could be identified (Figure 6, Patient 4). At the end of operation, a chest tube was placed through one port and the other ports were closed.



We controlled immediate postoperative pains using IV NSAID (Diclofenac ampule 90 mg) for 24 h every 8 h after surgery. And then we prescribed Oral NSAID (Zaltoprofen tablet 80 mg) for 3 days after every meal. Basically IV patient controlled anesthesia (PCA) was applied to every patient.
Results
The results of the operations performed on the 8 patients are shown in Table 1. Regarding patient characteristics, 6 of the 8 patients were male, and the average age was 51.7 years (range 34–85 years). Of the 8 resections, 5 were on the left side. The preoperative symptoms included wrist pain in 2 patients, upper extremity weakness in 2 patients, a tingling sensation in 2 patients, upper extremity edema in 1 patient, and a traumatic first rib fracture in 1 patient. Abnormal angiographic findings were observed in six patients (Cases 2, 3, 4, 6, 7, and 8), and one patient had abnormal findings in a nerve conduction velocity test (Case 1). Case 5 had flail chest and a fractured first rib just below the left subclavian artery.
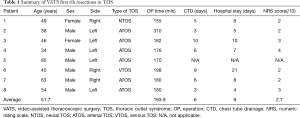
Full table
The average operative time was approximately 190 min. The average postoperative chest tube indwelling time was 6 days and the average duration of hospital stay after surgery was 9 days. The patient with trauma (Case 5) was excluded from the analysis of operative time, length of hospital stay, and chest tube indwelling time because the intraoperative fixation of the flail chest was combined with the first rib resection. No operative mortality occurred. One patient had a large amount of pleural effusion lasting for ten days after surgery (Case 3), whereas immediate postoperative venous thrombosis occurred in the patient with effort thrombosis (Case 6). This patient underwent fluoroscopic thromboembolectomy. The mean postoperative pain score was 2.7 points. During the follow-up period, no recurrent symptoms were observed in any of the provocation tests.
Discussion
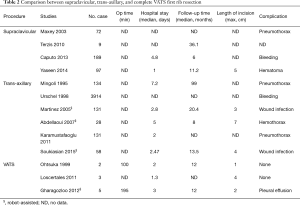
Extrathoracic first rib resection for TOS has been performed through supraclavicular or transaxillary incisions for approximately 50 years (5). The safety and usefulness of these approaches has already been established by a number of studies (8,10,14-21) (Table 2). The supraclavicular approach is one of the most reliable methods and is compatible with simultaneous scalenectomy. However, large wounds, hematomas (8), lymphatic leakage, lung herniation (22), nerve damage, and surgical failure (8) also can occur as complications of this approach. Transaxillary first rib resection is a procedure associated with smaller scars and reduced likelihood of injury to the muscles and nerves compared with the supraclavicular approach (7). For these reasons, the transaxillary approach is currently the most popular approach for removing the first rib. However, this approach still has some disadvantages, such as difficulty in visualizing the surgical field. To address these drawbacks, one recent study reported a transaxillary first rib resection using VATS through an intercostal port and a small axillary incision (21). However, extrathoracic axillary tissue and muscles must still be dissected to obtain the first rib. In cases of recurrent TOS after operation, the posterior approach can potentially be used for reoperation (23).
Full table
In the late 1990s, thoracoscopic techniques were developed and VATS was first attempted for first rib resection. Ohtsuka and colleagues succeeded in removing the first rib intrathoracically using an endoscopic drill under a video-assisted thoracoscope (24). Other studies reported that VATS first rib resection could be performed safely as a minimally invasive procedure using a double jointed bone cutter through extended intercostal wounds (25). Similarly, robot-assisted first rib resection was successfully performed through intercostal ports (26). In robotic cases and in the other techniques, a bone cutting instrument was applied through extended VATS ports to cut bone.
In this study, we performed VATS-IFRR exclusively through intercostal ports in all 8 cases. Moreover, all surgeries were performed without extending the intercostal ports. In contrast to another report of VATS using rib cutters through a 3–4 cm intercostal or axillary incision (21), we used long intervertebral disc rongeurs and Kerrison bone cutters to remove the rib pieces. One study found that specialized endoscopic bone cutters are optimal for VATS first rib resection (27). However, in clinical settings lacking specialized instruments, Kerrison bone cutters and other conventional spinal surgical instruments can be used for VATS-IFRR.
No serious bleeding events or damage of adjacent structures occurred in any our cases. However, the VATS approach does have some limitations. If the subclavian vessels are injured during surgery, or if vascular resection and reconstruction are needed for arterial obstruction and aneurysm, it may be difficult to control bleeding under VATS. In addition, scalenectomy was not performed in any previous study, although VATS cervical rib resection was presented in a recent report. We believe that scalenectomy will be possible under VATS. Despite the aforementioned limitations, VATS-IFRR has several advantages.
First, the wound was cosmetically superior to those achieved with other approaches. This method also allows the operation to be performed using 3–4 VATS ports (5 or 10 mm). Moreover, all scars were located inside the axillary area.
Second, the pain was not severe. In VATS-IFRR, no other structure involved in the thoracic outlet, except the first rib, needed to be dissected. In addition, brachial plexus traction can be avoided, which reduces the resulting pain. In fact, almost all patients had a numeric rating scale (NRS) score of 4 or less (Table 1).
Third, the intrathoracic approach to first rib resection had several advantages. The whole length of the first rib can be better visualized, thus enabling more complete resection. Moreover, the major muscles—including neck and back muscles—did not need to be divided. Furthermore, no injury occurred in the supraclavicular, phrenic, long thoracic, or brachial plexus nerves.
In addition, this method could be performed without causing additional wounds to the patient with trauma. The supraclavicular and transaxillary approaches have previously been used to treat TOS caused by trauma, such as first rib fractures (3). However, we propose that VATS-IFRR (without additional incisions) is superior to the supraclavicular and axillary approaches with respect to pain control and other complications when multiple rib fixations and first rib resection are required simultaneously. We also compared the operative times of each method to those reported in the literature. Although we hypothesized that VATS would have a longer operative time, we found similar result for all methods. Cosmetically, the size of the wound is smaller in VATS than in other approaches. Furthermore, our study showed comparable results for VATS and the other methods with respect to symptom relief, pain, and duration of hospital stay after surgery.
The chest tube drainage duration may be a little bit longer than conventional thoracic surgery. Our criteria of removal of chest tube after surgery are less than 100 mL/day. It used to be measured at every 6 AM. Actually criteria of chest tube removal is various among institutes. We think our criteria are stricter than others.
Few studies have reported the application of VATS to first rib resection for TOS. The number of cases in our study was small and the duration of the follow-up period was short. Therefore, randomized controlled trials comparing VATS-IFRR with alternative methods need to be performed to fully validate the efficacy and safety of this technique. In conclusion, our data indicate that VATS-IFRR is a potential minimally invasive surgical option for treating selective cases of TOS.
Acknowledgements
The authors thank our clinical nursing specialists, Won Ju Lee, and Heung Ki Kim.
Funding: This paper was conducted by 2016 Korea University Ansan Hospital R&D support project through the support of Vice President for Medical Affairs of Korea University special research funds (No. K1613821).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethic Committee on Clinical Investigation for Human Research (IRB) at Korea University Ansan Hospital (Ansan, South Korea) (No. AS170309-002). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Roos DB. Experience with first rib resection for thoracic outlet syndrome. Ann Surg 1971;173:429-42. [Crossref] [PubMed]
- Roos DB. Congenital anomalies associated with thoracic outlet syndrome: Anatomy, symptoms, diagnosis, and treatment. Am J Surg 1976;132:771-8. [Crossref] [PubMed]
- Lee TY, Cho HM, Kim YJ, et al. A case of traumatic thoracic outlet syndrome. Korean J Thorac Cardiovasc Surg 2012;45:412-4. [Crossref] [PubMed]
- Crosby CA, Wehbe MA. Conservative treatment for thoracic outlet syndrome. Hand Clin 2004;20:43-9. vi. [Crossref] [PubMed]
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014;11:CD007218. [PubMed]
- Urschel HC Jr. Management of the thoracic-outlet syndrome. N Engl J Med 1972;286:1140-3. [Crossref] [PubMed]
- Roos DB. Transaxillary approach for first rib resection to relieve thoracic outlet syndrome. Ann Surg 1966;163:354-8. [Crossref] [PubMed]
- Yaseen Z, Baram A. Neurogenic thoracic outlet syndrome treatment by the supraclavicular approach. Asian Cardiovasc Thorac Ann 2014;22:193-6. [Crossref] [PubMed]
- Orlando MS, Likes KC, Mirza S, et al. A Decade of Excellent Outcomes after Surgical Intervention in 538 Patients with Thoracic Outlet Syndrome. J Am Coll Surg 2015;220:934-9. [Crossref] [PubMed]
- Terzis JK, Kokkalis ZT. Supraclavicular approach for thoracic outlet syndrome. Hand (N Y) 2010;5:326-37. [Crossref] [PubMed]
- Hwang J, Min BJ, Jo WM, et al. The parietal pleura of the first rib was opened using a 5-mm endoscopic grasper and either a hook-type electrocautery probe or an ultrasonic shear probe. Asvide 2017;4:295. Available online: http://www.asvide.com/articles/1607
- Hwang J, Min BJ, Jo WM, et al. The anterior costal cartilage and posterior arc of the first rib were cut and trimmed using a 4.5-mm width Kerrison punch. Asvide 2017;4:296. Available online: http://www.asvide.com/articles/1608
- Hwang J, Min BJ, Jo WM, et al. Subclavian artery, subclavian vein, anterior scalene muscle, middle scalene muscle, and first rib were separated using endoscopic graspers and a hook-type electrocautery probe (or ultrasonic shear probe) under the traction of the first rib. Asvide 2017;4:297. Available online: http://www.asvide.com/articles/1609
- Maxey TS, Reece TB, Ellman PI, et al. Safety and efficacy of the supraclavicular approach to thoracic outlet decompression. Ann Thorac Surg 2003;76:396-9; discussion 9-400. [Crossref] [PubMed]
- Caputo FJ, Wittenberg AM, Vemuri C, et al. Supraclavicular decompression for neurogenic thoracic outlet syndrome in adolescent and adult populations. J Vasc Surg 2013;57:149-57. [Crossref] [PubMed]
- Mingoli A, Feldhaus RJ, Farina C, et al. Long-term outcome after transaxillary approach for thoracic outlet syndrome. Surgery 1995;118:840-4. [Crossref] [PubMed]
- Urschel HC Jr, Razzuk MA. Neurovascular compression in the thoracic outlet: changing management over 50 years. Ann Surg 1998;228:609-17. [Crossref] [PubMed]
- Martinez BD, Wiegand CS, Evans P, et al. Computer-assisted instrumentation during endoscopic transaxillary first rib resection for thoracic outlet syndrome: a safe alternate approach. Vascular 2005;13:327-35. [Crossref] [PubMed]
- Abdellaoui A, Atwan M, Reid F, et al. Endoscopic assisted transaxillary first rib resection. Interact Cardiovasc Thorac Surg 2007;6:644-6. [Crossref] [PubMed]
- Karamustafaoglu YA, Yoruk Y, Tarladacalisir T, et al. Transaxillary approach for thoracic outlet syndrome: results of surgery. Thorac Cardiovasc Surg 2011;59:349-52. [Crossref] [PubMed]
- Soukiasian HJ, Shouhed D, Serna-Gallgos D, et al. A video-assisted thoracoscopic approach to transaxillary first rib resection. Innovations (Phila) 2015;10:21-6. [Crossref] [PubMed]
- Su F, Zoole JB, Thompson RW, et al. Lung herniation after supraclavicular thoracic outlet decompression. Ann Thorac Surg 2012;93:1720-2. [Crossref] [PubMed]
- Urschel HC Jr, Razzuk MA, Albers JE, et al. Reoperation for recurrent thoracic outlet syndrome. Ann Thorac Surg 1976;21:19-25. [Crossref] [PubMed]
- Ohtsuka T, Wolf RK, Dunsker SB. Port-access first-rib resection. Surg Endosc 1999;13:940-2. [Crossref] [PubMed]
- Loscertales J, Congregado M, Jimenez Merchan R. First rib resection using videothorascopy for the treatment of thoracic outlet syndrome. Arch Bronconeumol 2011;47:204-7. [PubMed]
- Gharagozloo F, Meyer M, Tempesta BJ, et al. Robotic en bloc first-rib resection for Paget-Schroetter disease, a form of thoracic outlet syndrome: technique and initial results. Innovations (Phila) 2012;7:39-44. [Crossref] [PubMed]
- McKenna RJ, Mahtabifard A, Swanson SJ. Atlas of Minimally Invasive Thoracic Surgery (VATS): Expert Consult. 1st Edition. Saunders, 2010.


