Calcium oxalate crystal deposition in a patient with Aspergilloma due to Aspergillus niger
Introduction
The presence of calcium oxalate and black deposits in lung parenchyma is considered as evidence of Aspergillus niger infection (1,2). Here, we describe a patient with aspergilloma due to A. niger infection in whom rapidly deteriorating respiratory failure was accompanied by bacterial infection and pathological findings of black deposits characteristic of calcium oxalate.
Case report
A 72-year-old man was transferred to our hospital with hemoptysis that had persisted for 2 weeks. His medical history included total gastrectomy for gastric cancer 8 years previously. Nine months before arrival at our hospital, thoracic computed tomography (CT) during a routine medical checkup showed old inflammatory and cystic changes in the right upper lung lobe (Figure 1A,B), but not in the lower lung lobes (Figure 1C). The patient had been in his usual state of health until 1 month before admission, when he had presented with low-grade fever and productive cough. A chest X-ray showed dense infiltration in the right upper lung field (data not shown). Right upper pneumonia was tentatively diagnosed and he was administered with oral levofloxacin (500 mg/day) and clarithromycin (400 mg/day). No signs of either clinical or radiological improvement were evident after 1 week. At the same time, thoracic CT (Figure 1D,E) revealed a 2.5-cm lesion resembling a fungal ball with air space consolidation in the right upper lobe (Figure 1F). He was thus diagnosed with pulmonary aspergilloma or chronic necrotizing pulmonary aspergillosis (CNPA) and oral itraconazole (200 mg/day) and intravenous micafungin (150 mg/day) were administered. However, this strategy did not affect his respiratory status and the appearance of hemoptysis prompted admission to a local hospital. Although sputum cultures were negative for fungi and bacteria including acid-fast strains, alternative intensive treatments targeting Aspergillus infection (aspergilloma or CNPA) or bacterial pneumonia were started with intravenous liposomal amphotericin B (L-AMPHB) (5 mg/kg/day) plus meropenem (3 g/day). However, his respiratory status deteriorated over the next 2 weeks, and he was transferred to our hospital.
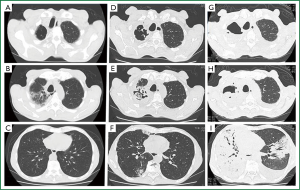
He appeared very ill upon arrival, with a blood pressure of 92/48 mmHg, respiratory rate of 30 breaths/min, body temperature of 38 °C, pulse rate of 56/min and oxygen saturation of 95% with a face mask delivering 5 L/min. A physical examination revealed conjunctival anemia, decreased breath sounds all over the right lung and coarse crackles in the left middle to bilateral lower lung fields. Laboratory data showed anemia (8.7 mg/dL), severe thrombocytopenia (4.2×104/L), anti-Aspergillus antibody and elevated C-reactive protein (16.7 mg/dL), but two sets of blood cultures, serum endotoxin antigen, galactomannan and β-D-glucan were negative. Thoracic CT imaging and bronchography upon admission (Figure 1G,H,I) showed that the right lung and left middle lobe were replaced by a massive consolidation and bilateral pleural effusion. Despite continued L-AMPHB and meropenem, the patient died of progressive respiratory failure on hospital day 2.
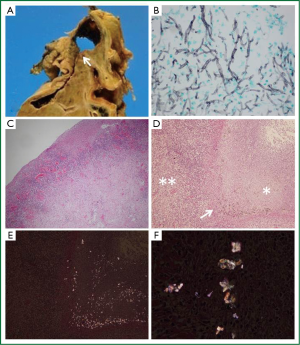
Autopsy specimens showed a cavity of 5 cm in diameter in the right upper lobe containing 2.5 cm of dark brown putrid material (Figure 2A, arrow). Grocott’s methenamine silver stain showing abundant branched filamentous hyphae (Figure 2B) suggested Aspergillus infection. However, odorous materials within a confined space were only cultured for Pseudomonas aeruginosa and Enterobacter cloacae. Furthermore, the cavity tightly adhered to the pleura and cultures of the right pleural effusion were also positive for P. aeruginosa, implicating parapneumonic effusion. Hematoxylin and eosin (H-E) stain did not reveal hyphal invasion in contiguous lung parenchyma, vessels and cavitary walls, which rather suggested aspergilloma, but not CNPA. Importantly, the cavitary wall contained numerous inflammatory cells (Figure 2C,D, double asterisks) without hyphal invasion, and H-E stain of residual necrotic tissue in the cavity (Figure 2D, asterisk) containing a black pigment (Figure 2D, arrow). Birefringent calcium oxalate was located adjacent to black deposits on partially crossed polaroids (Figure 2E). The calcium oxalate was clearer on fully crossed polaroids (Figure 2F). Interestingly, small amount of calcium oxalate was also noted in the left lung parenchyma (Figure not shown), which suggesting of transbronchial spread. Thereafter, the polymerase chain reaction showed that fungal ball specimens were positive for A. niger. The patient was thus diagnosed with aspergilloma due to A. niger and cavitary co-infection with P. aeruginosa or meropenem-sensitive Enterobacter cloacae.
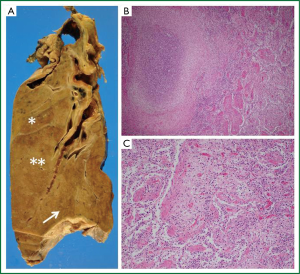
A pathological evaluation showed lung abscesses with organizing pneumonia in lung parenchyma surrounding the cavity in the right upper (Figure 3A, asterisk) and middle lobes (Figure 3A, double asterisks and B). Hyaline membranes and interstitial fibroblast proliferation were evident in the right lower lobe (Figure 3A, arrow), suggesting diffuse alveolar damage at the organizing stage (Figure 3C). This pathological feature was similar to that of the left lingular segment (data not shown). No calcium oxalate was detected in the left lingular segment and bacterial infection was not evident elsewhere in the lung parenchyma except for cavitary materials.
Discussion
Wehmer first described oxalic acid as a fermentation product of A. niger 1981 (3). Both A. niger and A. fumigatus produce oxalic acid, which precipitates as calcium oxalate by reacting with tissue fluids or blood (4). Oxalic acid is toxic and can damage localized tissue and/or blood vessels. Although calcium oxalate is not always detected in patients with Aspergillus infection (3), its presence is considered a characteristic of A. niger infection (5), and black pigment and calcium oxalate crystals can be easily identified in routine sputum examinations (6). However, the heaviest deposits in our patient were found within cavitary material located in an area where A. niger was undetectable. A. niger has large, globose, dark brown-to-black conidial heads that contain fungal spores, which accounts for the black sputum and pleural effusions of infected patients (7). The conidia of black Aspergillus species have brown and green pigments that absorb light across the entire visible spectrum. However, the precursor of the low-molecular-weight brown melanin pigment has not been characterized in detail (8). Scattered black pigments corresponded to conidial heads in cavitary material located in an area adjacent to calcium oxalate in our patient. Nime et al. (3) found black-pigmented A. niger conidial structures mainly adjacent to mycelia or calcium oxalate, but each could be separately located (3,9), as found in our patient. The radiological findings of our patient seemed to indicate a transitional phase from aspergilloma to CNPA (semi-invasive aspergillosis), but Aspergillus spp. were not cultured from the fungal ball material obtained from the cavity. Concurrent positive and negative antigen findings are common in CNPA as well as in aspergilloma.
Others have shown that A. niger can produce extracellular lipase in vitro under low pH (10), which is generated by the presence of calcium oxalate. Yoshida (9) created an immune-compromised rat model using immunosuppressive drugs and A. niger infection delivered via intratracheal inoculation. That study indicated that lung injury was probably caused by crystal production, rather than by direct A. niger invasion. A. niger, and to a lesser degree other Aspergillus species, release oxalic acid as a mycotoxin, which is formed as a side product of the tricarboxylic cycle by enzymatic hydrolysis of oxaloacetate by ocaloacetate acetylhydrolase. Previous report (11) described that Aspergillus-induced calcium oxalate crystal deposition can cause massive pulmonary hemorrhage and subsequent multiple organ failure with no evidence of tissue or blood vessel invasion by A. niger mycelia, which confined to cavity. Thus, calcium oxalate might have provoked severe inflammation of the lung parenchyma adjacent to the cavity, even after hyphae were killed or rendered less active by antifungal therapy. Thus, identification of calcium oxalate and black pigments in pathological specimens of the lungs and other organs could be pivotal clinical clues indicating infection with A. niger (12).
How organizing pneumonia is generated in patients with Aspergillus infection remains unknown, but a few studies of semi-invasive pulmonary aspergillosis have found that organizing pneumonia can develop in areas without hyphae (13,14). Most Aspergillus infections are attributed to Aspergillus fumigatus, followed by Aspergillus flavus and Aspergillus terreus. A. niger is a less common etiology of invasive disease, and it rarely causes pneumonia (13). From this viewpoint, P. aeruginosa or Enterobacter cloacae might have caused the respiratory failure in our patient, but serum endotoxin was not elevated, the results of two sets of blood cultures were negative, and no other organs had features typical of bacterial infection such as microabscesses, thrombosis, and infarction, suggesting that A. niger was the cause.
This experience highlighted the difficulty and/or dilemma involved in diagnosing pulmonary aspergillosis because only surgical resection can provide a definitive diagnosis, and the presence of calcium oxalate and black deposits in a pathological assessment indicate A. niger infection. The findings from this patient showed that in addition to calcium oxalate, an unknown somatic antigen of A. niger might also cause severe lung inflammation adjacent to an aspergilloma or other lung areas presenting as organizing pneumonia or diffuse alveolar damage even in patients with essentially normal or previously compromised immune status.
Acknowledgements
This work was supported by the Health Science Research Grants for Research On Emerging and Reemerging Infectious Diseases (H22ShinkouIppan8 and H23ShinkouIppan018)and Measures for Intractable Diseases (H20Nannchi Ippann35) from the Ministry of Health, Labor and Welfare of Japan, a grant from the Strategic Basis on Research Grounds for Nongovernmental Schools at Heisei 20th, the Strategic Research Foundation Grantaided Project for Private Schools at Heisei 23rd, and KAKENHI (#24790364) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Disclosure: The authors declare no conflict of interest.
References
- Saraya T, Light RW, Takizawa H, et al. Black pleural effusion. Am J Med 2013;126:641.e1-6.
- Nakagawa Y, Shimazu K, Ebihara M, et al. Aspergillus niger pneumonia with fatal pulmonary oxalosis. J Infect Chemother 1999;5:97-100. [PubMed]
- Nime FA, Hutchins GM. Oxalosis caused by aspergilus infection. Johns Hopkins Med J 1973;133:183-94. [PubMed]
- Kimmerling EA, Fedrick JA, Tenholder MF. Invasive Aspergillus niger with fatal pulmonary oxalosis in chronic obstructive pulmonary disease. Chest 1992;101:870-2. [PubMed]
- Kurrein F, Green GH, Rowles SL. Localized deposition of calcium oxalate around a pulmonary Aspergillus niger fungus ball. Am J Clin Pathol 1975;64:556-63. [PubMed]
- Kuwabara H, Shibayama Y. Pulmonary aspergilloma with prominent oxalate deposition. Indian J Pathol Microbiol 2012;55:589-90. [PubMed]
- Metzger JB, Garagusi VF, Kerwin DM. Pulmonary oxalosis caused by Aspergillus niger. Am Rev Respir Dis 1984;129:501-2. [PubMed]
- Jørgensen TR, Park J, Arentshorst M, et al. The molecular and genetic basis of conidial pigmentation in Aspergillus niger. Fungal Genet Biol 2011;48:544-53. [PubMed]
- Yoshida K. The correlation between tissue injury and calcium oxalate crystal production in rat’s lung with experimental Aspergillus niger infection. Kansenshogaku Zasshi 1998;72:621-30. [PubMed]
- Marini A, Imelio N, Picó G, et al. Isolation of a Aspergillus niger lipase from a solid culture medium with aqueous two-phase systems. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879:2135-41. [PubMed]
- Roehrl MH, Croft WJ, Liao Q, et al. Hemorrhagic pulmonary oxalosis secondary to a noninvasive Aspergillus niger fungus ball. Virchows Arch 2007;451:1067-73. [PubMed]
- Vaideeswar P, Sakhdeo UM. Pulmonary aspergilloma with renal oxalosis: fatal effect at a distance. Mycoses 2009;52:272-5. [PubMed]
- Person AK, Chudgar SM, Norton BL, et al. Aspergillus niger: an unusual cause of invasive pulmonary aspergillosis. J Med Microbiol 2010;59:834-8. [PubMed]
- Ishifujia T, Takakia M, Tsuchihashib Y, et al. A case of acute progressive respiratory failure resulting from pulmonary aspergillosis with pulmonary oxalosis. AJRS 2013;2:370-4.


