The impact of flexible endoscopy in esophageal surgery
Introduction
Flexible endoscopy has recently become a unique tool for esophageal surgeons in the aim of reducing morbidity and advancing the minimally invasive era. The early legacy of natural orifice transluminal endoscopic surgery has yielded a number of tools that allow complete endoscopic treatment of cases that a decade ago could only be treated with surgery. The two main areas within esophageal surgery where endoscopic approaches have gained attention are treatment of achalasia with endoscopic myotomy and esophageal preservation in esophageal cancer. This manuscript reviews the main achievements in both fields and summarizes the main technical features. The impact on the field is enormous and compulsive training in flexible endoscopy should be mandatory for esophageal surgeons.
Peroral endoscopic myotomy (POEM)
Achalasia is a rare esophageal motility disorder. It is characterized by the loss of peristalsis of the esophageal body and the absence or lack of adequate relaxation of the lower esophageal sphincter (LES), due to a selective alteration of the inhibitory neurons at that level. Therapeutic alternatives include surgical myotomy and endoscopic methods such as balloon dilatation and botulinum toxin injection aimed at weakening or relaxing the LES (1).
In recent years peroral endoscopic myotomy appeared as a new alternative and has been widely adopted due to the low morbidity and encouraging results in the different series (2).
The initial reports of the different published series present the POEM as an effective and low morbidity alternative for the treatment of classical achalasia (3).
There are also reports of good POEM results in cases of other motor disorders such as diffuse esophageal spasm, hypertensive LES, nutcracker or jackhammer esophagus where the outcome of laparoscopic myotomy is not as effective. POEM is recommended in these diffuse motor disorders and not the classical Heller myotomy, since it allows a more extended myotomy from the proximal esophagus (4).
Patients with prior therapies make the procedure more difficult, but it can safely be done in experienced groups. In patients who have undergone a Heller myotomy, a new myotomy on the anterior face is subject to a high risk of mucosal opening with the possible complications that this entails. POEM performed in hour 7 (posterior face), eliminates this risk and adds the possibility of a better myotomy (5). This is probably the clearest and most relevant indication for this method.
POEM technique
In order to perform an endoscopic myotomy, it is generally necessary to have a working overtube, a high resolution endoscope, and a CO2 insufflator. Tools include a semi-rigid cap, an injector, a coagulation grasper and an endoscopic knife with a monopolar power source with spray mode.
The site of the mucosal incision is located at least 15 cm distant from the UEG and ideally at hour 2 or 5. Saline solution stained with indigo carmine is instilled in the submucosal layer to lift the mucosa and allow safe entering into the submucosal space. Under direct vision, a submucosal tunnel is created along the esophagus, through the EGJ up to 2–3 cm distal on the gastric side. Myotomy starts 5 cm below the mucosal incision and extends to the rest of the tunnel. Although the precise indication of the myotomy is only of the circular layer, there are studies reporting the safety of full-thickness myotomy (6). Closure of the mucosal incision is safely performed with endoclips, usually in number of 4 (Figure 1).
Results
A comprehensive review and tabulation of efficacy results with papers published up to the beginning of 2014 reports excellent efficacy rates (90–100% at 3–12 months), except in the European multicentric series, where it was 82% in patients who completed 1-year follow-up. Efficacy was measured using the parameter of decreased Eckardt Score (ES) to 3 or less (7).
Studies have shown that centers need to perform at least 20–40 procedures to complete the learning curve (8,9). In the last 2 years, new publications provided data with longer-term follow-up. Four Western series of pioneer centers in Portland (USA), Chicago (USA), Mineola (USA), and Rome (Italy) with 100, 41, 93, and 100 patients respectively, reported clinical success rates of 92%, 93%, 96%, 94% at the mean follow-up of 21.5, 12, 22, and 11 months respectively (Table 1). In the largest series of cases to date, Inoue et al. reported results in 500 patients, with 105 patients with more than three years of follow-up (10). The procedure was successfully performed in all patients. Moderate adverse events occurred in 3.2% including pneumothorax, bleeding, mucosal lesions, postoperative hematomas, pleural effusion, and inflammation of minor omentum. Most were managed conservatively. There were no serious adverse events. Clinical success was achieved in 91.7%. At endoscopic follow-up, 65% had signs of reflux esophagitis, but only 17% of patients complained of GERD symptoms. At three years, overall success remained high in 88.5%, with GERD symptomatic in 21% and signs of reflux esophagitis in 56%. All reflux symptoms were effectively controlled with proton pump inhibitors (10).

Full table
Reflux disease (GERD) and POEM
The problem of GERD after POEM is of great interest because it is rapidly displacing Heller’s myotomy as the first line therapy for achalasia in most patients. To date, only four series have presented substantial data on the evaluation of GERD in their patients using all three methods (systematic symptom assessment, endoscopic evaluation and outpatient pH study) (11,12). These studies found that 27–59% of patients had endoscopic reflux symptoms (mainly mild esophagitis class A or B of Los Angeles), 29–38% had abnormally high acid exposure in the pH studies, and 15–23% had frequent reflux symptoms. These patients have been treated effectively with PPI. It should be noted that the fundoplication of Dor or Toupet performed in conjunction with a laparoscopic Heller myotomy in patients with achalasia has modest efficacy. High-quality studies of laparoscopy centers have shown that 18–42% of patients present abnormal exposure to the acid in the postoperative period, similar to that observed in the post-POEM study (13,14). It is not clear why the rate of GERD after POEM is not substantially greater than after a Heller myotomy combined with fundoplication. It may be due to no hiatal dissection during POEM compared to extensive dissection of the hiatus during a standard myotomy. This extensive dissection disrupts important ligaments of the esophagus, which are thought to contribute to the maintenance of the angle of His, which is the main barrier remaining after myotomy. This mechanism is not altered during POEM.
Our experience
Fifty cases of POEM were analyzed prospectively between December 2013 and August 2016. The mean follow-up was 10 months (6/32). The extension was limited until obtaining a Hill type II valve and never exceeded 2 cm. Endpoints included the clinical outcome measured by the Eckardt score (ES), presence of symptomatic reflux of the related Quality of Life Questionnaire (GERD HQRL), need for PPI, and esophagitis discarded by endoscope. The poem was completed in 100% of the patients. Follow-up was 100%. Efficacy (ES ≤3) was 47/50 (94.2%) at a short-term follow-up and 44/50 (88.6%) at long-term follow-up, with a mean ES decline from 9 to 1.2 (P=0.0001). There were intraoperative complications n=2 (mucosal bleeding and perforation) and immediate post-operative n=1 (massive capnothorax) managed in a conservative procedure that did not require conversion or reintervention. The average number of days of hospitalization was 1.3 days. The cases of symptomatic reflux were 10/50 (20%) with signs of endoscopic esophagitis in 4/50 (8%). Patients currently requiring PPIs are 4/50 (8%). Additional treatment (endoscopic dilatation) was performed in 10/50 cases (20%).
Conclusions
The POEM is a safe and effective method that allows thinking about a paradigm shift regarding laparoscopic myotomy. Encouraging results and low morbidity yield to a faster recovery of the patient that stimulates adoption of the procedure. The need to have a multidisciplinary team with extensive experience in therapeutic endoscopy makes it advisable to limit this procedure to centers of reference and high volume in this disease.
Endoscopic therapies for early esophageal adenocarcinoma
Esophageal adenocarcinoma is increasing at a rate greater than any cancer in the Western hemisphere. Treatment most often requires esophageal resection, a procedure that is associated with considerable morbidity and mortality (15,16). In the last two decades, efforts have been made to diagnose esophageal cancer at an earlier stage so as to facilitate preservation of the esophagus and improve long-term survival and quality of life (17-19). With minimally invasive surgical and endoscopic techniques evolving rapidly, there has been a substantial paradigm shift in the management of early stage neoplasia in Barrett’s esophagus (BE) comprising high-grade dysplasia (HGD), intramucosal and, in some cases, submucosal carcinoma (20,21). The availability of more therapeutic options interjects an increasing degree of complexity regarding the optimal therapeutic algorithm to be employed.
Previously, the majority of patients with early-stage esophageal neoplasia would undergo surgical resection of the esophagus in order to eliminate the risk of occult disease progression and ensure long-term survival. However, in these cases, esophageal resection was performed at the expense of the related to having a gastric interposition (i.e., regurgitation, early satiety, stricture, aspiration) (22). Organ preservation, defined as any endoluminal procedure used in an attempt to completely eradicate disease while preserving the anatomic structure and physiology of the esophagus has now become an option for patients with early stage neoplasia (23).
Recently, the American Gastroenterological Association (13) published a position statement on the management of BE with an analysis of available evidence to support decision-making related to diagnosis, screening and treatment of BE (24). In parallel, a consensus statement was created by a group of experts on the management of BE and early cancer including diagnosis, staging and therapeutic approaches (21).
Radiofrequency ablation (RFA)
RFA using the HALO system (Covidien Inc., Mansfield, MA) is the most commonly performed ablation therapy. This system includes either an ablation balloon catheter (HALO360) for circumferential ablation or an endoscope-mounted device (HALO90,60,Ultra) for focal ablation to deliver a high-power short burst of ablative energy to the abnormal esophageal epithelium.
The energy delivered provides uniform treatment to a depth of 500 µm. The depth of treatment is therefore limited to the mucosal layer and the risk of stricture formation is significantly reduced compared to other ablative techniques (21,25-30). Success rates in eradicating dysplasia are reported to be over 90% with near complete eradication of intestinal metaplasia in controlled trials. The overall complication rate ranges from 3–7% with the most common being stricture (31). Durability of the method has been shown to be over 85% at 3 years and disease progression has been reported to be 1.37% per patient year in 127 patients with a 3-year follow-up (29). Limitations of this method include the lack of sample retrieval for histology analysis and the possibility of leaving undetected buried glands (32,33). In this manuscript we review the main endoscopic tools or procedures that enable organ preservation and discuss its performance.
Endoscopic mucosal resection (EMR)
Currently, EMR is used as both a diagnostic and a therapeutic tool. The endoscopic cap resection technique and the ligate-and-cut technique are the most commonly used methods for EMR. A randomized trial to compare these 2 techniques has shown similar efficacy. EMR is usually indicated for tumors/nodules <2 cm in diameter (34-38). Long-term success rates are 96.6% in specialized centers with an 84% 5-year survival. Metachronous lesions developed during follow-up in up to 20% of patients (39). Limitations include the piecemeal resection that can hinder the histology analysis particularly when multifocality is present, stricture after extended resections (>50% circumference) and risk of perforation (40).
Endoscopic submucosal dissection (ESD)
ESD is an advanced endoscopic resection technique for en-bloc resection of lesions larger than 2 cm in diameter, thus providing more accurate histologic assessment for the lateral and deep margins of discrete nodules. In certain instances, this technique reduces the occurrence of metachronous lesions compared to EMR (41,42). In more advanced settings, the ESD technique may be extended to a circumferential sleeve mucosal resection to remove the entire abnormal epithelium, followed by a biological scaffold deployment for stricture prevention. This approach has been reported in a single center experience (23,43,44).
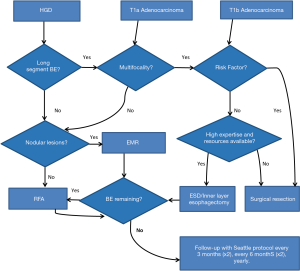
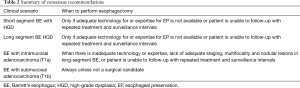
There is consensus that esophageal preservation could be attempted in patients with any length of HGD and in elderly patients with early cancer without submucosal invasion (T1a). In young and middle-aged patients with T1a adenocarcinoma, the esophageal preservation strategies remain controversial and should only be considered based on the expertise of each center, and on the availability of the appropriate technology. It is likely that with further follow-up on safety and durability of endoscopic therapies, esophageal preservation in these patients will become more broadly accepted. However, in patients with submucosal invasion (T1b), the consensus is that esophageal preservation has a limited role and surgical resection remains the preferred option due to the high probability of lymph node metastases in this patient group (Table 2) (45).
Full table
In order to attempt esophageal preservation it is advised that centers should be prepared with state-of-the art equipment and technology for interventional endoscopic procedures, as well as trained physicians with a high volume practice in the esophageal field. Gastroenterology, pathology, and foregut surgery units that work in a multidisciplinary manner are beneficial when dealing with these complex management algorithms. Recently, a multidisciplinary consensus from a group of experts was published for the management of BE and early stage esophageal adenocarcinoma ranging from diagnostic to therapeutic implications (7). They have provided over 80 consensus statements for all topics. Specifically for treatment of early stage neoplasia, many of their conclusions reached >80% agreement in support of the consensus in this manuscript. Briefly, it is stated that “For patients with HGD in an endoscopically visible abnormality, endoscopic resection is essential for proper diagnosis and staging. Endoscopic treatment should be preferred over surgical treatment for the management of most patients with BE with HGD and endoscopic treatment of HGD/T1m should only be performed in tertiary referral care centers after proper training of the endoscopists and pathologists involved” (7). The patient’s condition is also important when making decisions regarding the preservation of the esophagus and it should be assessed from several areas such as performance status and nutritional state.
We acknowledge that the pathology report is the main tool to predict risk of lymph node involvement in early stage neoplasia. It has been shown that lack of lymphovascular invasion, depth of invasion up to 500 µm (intramucosal), and well to moderately well differentiated adenocarcinoma are associated with very low risk of node metastasis and are ideal candidates for esophageal preservation (42,43). Although no clinical studies were published comparing esophagectomy versus endoscopic therapies for early stage cancer, a recent systematic review has found that only 2% of the patients with T1a were reported to have lymph node metastasis in the esophagectomy specimen which compares to the mortality rate of the esophagectomy in the best centers. Given the high morbidity of the procedure and the fact that cure cannot be warranted even after esophagectomy in patients that already have lymphatic spread, the authors conclude that risk of lymph node metastasis does not warrant the choice of esophagectomy over endoscopic therapies (44).
Functional status and co-morbidity is another relevant issue when a decision on a preservation strategy is ambiguous. Patients with very low surgical risk that have lesions where complete endoscopic eradication is unlikely due to technical or anatomical reasons should be referred to surgery instead of attempting preservation. Finally, the patient’s socioeconomic environment and his/her ability to follow-up treatment guidelines are very relevant at the time of making a decision. Any endoscopic therapy requires strict acid suppression therapy and intensive surveillance. Also, repeated interventions will be required. This needs to be discussed with patients prior to initiation of the treatment. Preservation should only be attempted in those patients who have full access to a complete health system and are willing and able to maintain a follow-up treatment over the course of several years. This is, at least, until further evidence on the need of follow-up is published which better defines increasing surveillance intervals (Figure 2) (7).
Conclusions
In conclusion, endoscopic advances in diagnostic and therapeutic arenas allow for organ preservation in most settings of early stage neoplasia of the esophagus provided that the clinical setting and physician’s expertise are prepared for this approach and the patient understands the implications of this decision. Thorough discussions with the patient on therapeutic options should precede any procedure and active involvement of the patient in the final decision is strongly encouraged. Surgical treatment remains the standard of care for invasive carcinoma, a paradigm that will shift as we learn which approaches prove to be safe and effective over long-term follow-up.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mayberry JF. Epidemiology and demographics of achalasia. Gastrointest Endosc Clin N Am 2001;11:235-48. v. [PubMed]
- Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265-71. [Crossref] [PubMed]
- NOSCAR POEM White Paper Committee., Stavropoulos SN, Desilets DJ, et al. Per-oral endoscopic myotomy white paper summary. Gastrointest Endosc 2014;80:1-15.
- Swanström LL, Rieder E, Dunst CM. A stepwise approach and early clinical experience in peroral endoscopic myotomy for the treatment of achalasia and esophageal motility disorders. J Am Coll Surg 2011;213:751-6. [Crossref] [PubMed]
- Stefanidis D, Richardson W, Farrell TM, et al. SAGES guidelines for the surgical treatment of esophageal achalasia. Surg Endosc 2012;26:296-311. [Crossref] [PubMed]
- Li QL, Chen WF, Zhou PH, et al. Peroral endoscopic myotomy for the treatment of achalasia: a clinical comparative study of endoscopic full-thickness and circular muscle myotomy. J Am Coll Surg 2013;217:442-51. [Crossref] [PubMed]
- Von Renteln D, Fuchs KH, Fockens P, et al. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology 2013;145:309-11.e1-3.
- Kurian AA, Dunst CM, Sharata A, et al. Peroral endoscopic esophageal myotomy: defining the learning curve. Gastrointest Endosc 2013;77:719-25. [Crossref] [PubMed]
- Patel KS, Calixte R, Modayil RJ, et al. The light at the end of the tunnel: a single-operator learning curve analysis for per oral endoscopic myotomy. Gastrointest Endosc 2015;81:1181-7. [Crossref] [PubMed]
- Inoue H, Sato H, Ikeda H, et al. Per-Oral Endoscopic Myotomy: A Series of 500 Patients. J Am Coll Surg 2015;221:256-64. [Crossref] [PubMed]
- Stavropoulos SN, Modayil RJ, Brathwaite CE, et al. 181 Outcomes of a 5-Year, Large Prospective Series of Per Oral Endoscopic Myotomy (POEM). Emphasis on Objective Assessment for GERD and Luminal Patency. Gastrointest Endosc 2015;81:AB118-9. [Crossref]
- Familiari P, Greco S, Gigante G, et al. Gastroesophageal reflux disease after peroral endoscopic myotomy: Analysis of clinical, procedural and functional factors, associated with gastroesophageal reflux disease and esophagitis. Dig Endosc 2016;28:33-41. [Crossref] [PubMed]
- Kumagai K, Kjellin A, Tsai JA, et al. Toupet versus Dor as a procedure to prevent reflux after cardiomyotomy for achalasia: results of a randomised clinical trial. Int J Surg 2014;12:673-80. [Crossref] [PubMed]
- Rawlings A, Soper N, Oelschlager B, et al. Laparoscopic Dor versus Toupet fundoplication following Heller myotomy for achalasia: results of a multicenter, prospective, randomized-controlled trial. Surg Endosc 2012;26:18-26. [Crossref] [PubMed]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94. [PubMed]
- Orringer MB, Marshall B, Chang AC, et al. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg 2007;246:363-72; discussion 72-4. [Crossref] [PubMed]
- DeMeester SR. Evaluation and treatment of superficial esophageal cancer. J Gastrointest Surg 2010;14 Suppl 1:S94-100. [Crossref] [PubMed]
- DeMeester SR. Endoscopic mucosal resection and vagal-sparing esophagectomy for high-grade dysplasia and adenocarcinoma of the esophagus. Semin Thorac Cardiovasc Surg 2005;17:320-5. [Crossref] [PubMed]
- Amano Y, Kinoshita Y. Barrett esophagus: perspectives on its diagnosis and management in asian populations. Gastroenterol Hepatol (N Y) 2008;4:45-53. [PubMed]
- Shaheen NJ, Inadomi JM, Overholt BF, et al. What is the best management strategy for high grade dysplasia in Barrett's oesophagus? A cost effectiveness analysis. Gut 2004;53:1736-44. [Crossref] [PubMed]
- Bennett C, Vakil N, Bergman J, et al. Consensus statements for management of Barrett's dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012;143:336-46. [Crossref] [PubMed]
- D'Journo XB, Martin J, Ferraro P, et al. The esophageal remnant after gastric interposition. Dis Esophagus 2008;21:377-88. [Crossref] [PubMed]
- Witteman BP, Foxwell TJ, Monsheimer S, et al. Transoral endoscopic inner layer esophagectomy: management of high-grade dysplasia and superficial cancer with organ preservation. J Gastrointest Surg 2009;13:2104-12. [Crossref] [PubMed]
- Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 2011;140:1084-91. [Crossref] [PubMed]
- Semlitsch T, Jeitler K, Schoefl R, et al. A systematic review of the evidence for radiofrequency ablation for Barrett's esophagus. Surg Endosc 2010;24:2935-43. [Crossref] [PubMed]
- Pouw RE, Gondrie JJ, Curvers WL, et al. Successful balloon-based radiofrequency ablation of a widespread early squamous cell carcinoma and high-grade dysplasia of the esophagus: a case report. Gastrointest Endosc 2008;68:537-41. [Crossref] [PubMed]
- Pouw RE, Sharma VK, Bergman JJ, et al. Radiofrequency ablation for total Barrett's eradication: a description of the endoscopic technique, its clinical results and future prospects. Endoscopy 2008;40:1033-40. [Crossref] [PubMed]
- Krishnan K, Komanduri S, Cluley J, et al. Radiofrequency ablation for dysplasia in Barrett's esophagus restores beta-catenin activation within esophageal progenitor cells. Dig Dis Sci 2012;57:294-302. [Crossref] [PubMed]
- Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology 2011;141:460-8. [Crossref] [PubMed]
- Ganz RA, Overholt BF, Sharma VK, et al. Circumferential ablation of Barrett's esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc 2008;68:35-40. [Crossref] [PubMed]
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [Crossref] [PubMed]
- Titi M, Overhiser A, Ulusarac O, et al. Development of subsquamous high-grade dysplasia and adenocarcinoma after successful radiofrequency ablation of Barrett's esophagus. Gastroenterology 2012;143:564-6.e1. [Crossref] [PubMed]
- Gray NA, Odze RD, Spechler SJ. Buried metaplasia after endoscopic ablation of Barrett's esophagus: a systematic review. Am J Gastroenterol 2011;106:1899-908; quiz 909.
- Thomas T, Ayaru L, Lee EY, et al. Length of Barrett's segment predicts success of extensive endomucosal resection for eradication of Barrett's esophagus with early neoplasia. Surg Endosc 2011;25:3627-35. [Crossref] [PubMed]
- Thomas T, Singh R, Ragunath K. Trimodal imaging-assisted endoscopic mucosal resection of early Barrett's neoplasia. Surg Endosc 2009;23:1609-13. [Crossref] [PubMed]
- Inoue H. Treatment of esophageal and gastric tumors. Endoscopy 2001;33:119-25. [Crossref] [PubMed]
- Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett's esophagus. Gastrointest Endosc 2000;52:328-32. [Crossref] [PubMed]
- Pech O, Ell C. Early esophageal cancer: pro-endoscopic resection. Chirurg 2011;82:490-4. [Crossref] [PubMed]
- Groome M, Lindsay J, Ross PE, et al. Use of oesophageal stress response proteins as potential biomarkers in the screening for Barrett's oesophagus. Eur J Gastroenterol Hepatol 2008;20:961-5. [Crossref] [PubMed]
- Lewis JJ, Rubenstein JH, Singal AG, et al. Factors associated with esophageal stricture formation after endoscopic mucosal resection for neoplastic Barrett's esophagus. Gastrointest Endosc 2011;74:753-60. [Crossref] [PubMed]
- Van Den Eynde M, Jouret-Mourin A, Sempoux C, et al. Endoscopic mucosal or submucosal resection of early neoplasia in Barrett's esophagus after antireflux surgery. Gastrointest Endosc 2010;72:855-61. [Crossref] [PubMed]
- Inoue H. Endoscopic mucosal resection for the entire gastrointestinal mucosal lesions. Gastrointest Endosc Clin N Am 2001;11:459-78. [PubMed]
- Nieponice A, McGrath K, Qureshi I, et al. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc 2009;69:289-96. [Crossref] [PubMed]
- Badylak SF, Hoppo T, Nieponice A, et al. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A 2011;17:1643-50. [Crossref] [PubMed]
- Nieponice A, Badaloni AE, Jobe BA, et al. Management of early-stage esophageal neoplasia (MESEN) consensus. World J Surg 2014;38:96-105. [Crossref] [PubMed]