Differences between low and high grade fetal adenocarcinoma of the lung: a clinicopathological and molecular study
Introduction
Fetal adenocarcinoma of the lung (FLAC) features a morphology resembling that of a developing fetal lung and was firstly reported in 1982 by Kradin (1). FLACs have been further divided into low-grade fetal adenocarcinomas (L-FLACs) and high-grade fetal adenocarcinomas (H-FLACs) as these two categories exhibit different clinicopathological features and biological behaviors (2,3). It was reported that H-FLAC constituted 0.4% of all primary lung cancer, while FLAC accounted for an estimated 0.5% of all primary lung tumors (4,5). L-FLAC often presents in earlier stages and has been linked to lower mortality risks in contrast to H-FLAC.
Histologically, FLACs comprises glandular components with tubules lined by glycogen-rich, non-ciliated cells. The pattern resembles the morphology of a developing fetal lung. L-FLAC is characterized by complex glandular structures with low nuclear atypia and prominent morule formation, which resembles the developing fetal lung during the pseudoglandular phase (6-9). In contrast, H-FLAC shows predominant nuclear atypia while morule formation is rarely observed (2,4,10). Several studies have revealed that L-FLAC constantly exhibits aberrant β-catenin nuclear/cytoplasmic expression and frequent β-catenin gene mutations. On the contrary, H-FLAC shows a membranous staining for β-catenin while lacking β-catenin gene mutations (11-13). FLAC is uncommon, and only a few studies with limited cases on L-FLAC and H-FLAC have been published) (2,4,7-10). In the present study, we retrospectively examined 3 L-FLAC cases and 5 H-FLAC cases to provide further information on the clinicopathological and molecular features of FLAC in Chinese patients.
Methods
Samples
A total of 920 consecutive cases of primary lung adenocarcinoma subjected to surgical resection in Peking Union Medical College Hospital between January 2006 and December 2014 were recruited for screening. H&E slides were retrieved from the archives of the pathology department and reviewed independently by two experienced histopathologists without prior knowledge to the patients’ conditions. Cases that met the inclusion criteria for fetal adenocarcinoma were subjected to further analyses. The criteria used to determine FLAC was adopted from previous studies, with minor revisions, as follows: (I) L-FLAC consists of complex glandular structures lined with glycogen-rich columnar cells, with low nuclear atypia, morule formation, and mainly nuclear- and cytoplasmic-localized β-catenin; (II) H-FLAC exhibits complex acinar glands that consist of columnar tumour cells with supranuclear or subnuclear cytoplasmic clearing, large vesicular nuclei, prominent nucleoli, the absence of morules, broad areas of necrosis, and primarily membrane-localized β-catenin; and (III) L-FLAC is pure in pattern, whereas H-FLAC has at least 50% fetal morphology (6).
Demographic characteristics and clinical information, including age, sex, smoking history, therapeutic regimens, and follow-up information, were retrieved from medical records. Tissue sections were reviewed in detail to obtain histological features. The follow-up period ranged from 7 to 121 months. The study protocol has been approved by the Ethics Committee of Peking Union Medical College Hospital with the approval number S-424, and informed consent was obtained for all cases.
Morphology and immunohistochemistry
Four-micrometer-thick sections were cut from formalin-fixed, paraffin-embedded (FFPE) tissue blocks for immunohistological staining. Staining for AE1/AE3 (clone AE1/AE3, Dako), AFP (clone C3, Novocastra), ALK-D5F3 (clone D5F3, Ventana), β-catenin (clone 14, Ventana), Chromogranin A (clone 5H7, Novocastra), CK7 (clone RN7, Novocastra), EGFR (clone 5B7, Ventana), Her-2 (clone 4B5, Ventana), p53 (clone DO7, Ventana), Synaptophysin (clone 27G12, Novocastra), and TTF-1 (clone SPT24, Novocastra) was performed using a Ventana Benchmark XT instrument (Ventana, Tucson, AZ, USA). All other reagents were obtained from Ventana. Appropriate positive and negative controls were included in each batch of staining for all antibodies.
All samples were evaluated and scored by 2 pathologists. For EGFR and Her-2, the intensity of the staining was graded semi-quantitatively on a four point scale (0; 1+; 2+; 3+). Any tumor cell with membranous staining of any intensity equal to or higher than 1+ was considered positivity of the cell. The positivity of the case was defined as a percentage of positive tumor cells larger than 10%. Positive ALK protein expression was defined by the presence of strong granular cytoplasmic staining in tumor cells at any percentage of positive tumor cells, whereas the absence of strong granular cytoplasmic staining in tumor cells was designated as ALK-negative. As for p53, nuclear staining of >10% of tumor cells at any intensity was considered p53 overexpression. As for AE1/AE3, AFP, β-catenin, Chromogranin A, CK7, Synaptophysin, and TTF-1, positivity were defined by the staining in >1% of tumor cells at any intensity.
DNA extraction
Four-micrometer-thick sections were cut from FFPE tissue blocks. From each block, five consecutive sections were made. One section was subjected to H&E staining and reviewed by a histopathologist. The other four sections were then subjected to microdissection, from which tumor tissue was scraped off and transferred to an Eppendorf tube. DNA extraction was performed using the QIAamp FFPE DNA tissue kit (QIAgen, cat# 56404) following the manufacturer’s instructions. DNA was eluted using 50 µL buffer ATE supplemented in the kit.
Detection of EGFR/KRAS/PIK3CA/BRAF mutations
EGFR, K-Ras, PIK3CA, and B-Raf mutations were screened by real-time PCR-based assays (Beijing ACCB Biotech, Beijing, China). The assays covered 63 hotspot mutations, including 45 mutations in exons 18, 19, 20, and 21 of EGFR; 12 mutations in exons 2 and 3 of K-Ras; 5 mutations in exons 9 and 20 of PIK3CA; and B-Raf V600E. PCR cyclings were performed on a Mx3000P PCR instrument (Agilent, Santa Clara, CA, USA), with the following settings: 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Amplification curves were analyzed and interpreted following the manufacturer’s instructions.
Results
Clinicopathological characteristics
Among the 920 lung adenocarcinoma cases screened, we were able to identify eight cases that met the criteria for FLAC, constituting a percentage of 0.87%, among which 3 (0.32%) were L-FLACs and 5 (0.54%) were H-FLACs. Within the five H-FLACs, two were originally diagnosed as moderately differentiated adenocarcinomas and categorized as H-FLACs during the histological review of the present study. Clinicopathological Characteristics of each patients was detailed in Table 1.The ages of the L-FLAC patients ranged from 27 to 49 years (mean =36.3 years) while that of the H-FLAC patients ranged from 52 to 75 years (mean =60.4 years). A history of smoking was found in four H-FLAC cases (80%) but no L-FLAC cases. All 3 L-FLAC patients were at stage I, whereas two and three H-FLAC cases were designated to be stage I and III, respectively. All 3 L-FLAC patients remained alive during the follow-up period, and none of these patients exhibited local recurrence or metastatic disease. Three H-FLAC patients died of the disease, and 2 H-FLAC patients remained alive and disease-free during the follow-up period.

Full table
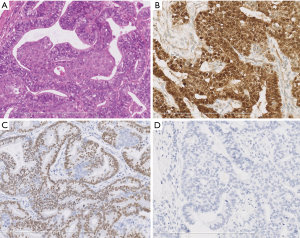
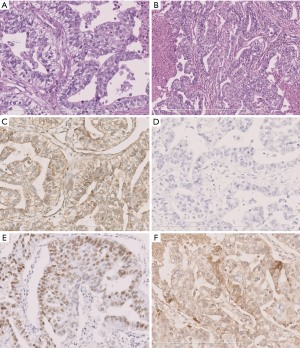
Typical histological and immunophenotypic features of L-FLAC and H-FLAC cases are shown in Figures 1,2, respectively. Histopathologically, all 3 L-FLACs were pure with regards to the pattern. The lining cells show clear cytoplasm and low nuclear atypia. Morules were observed in all 3 cases (Figure 1A). Necrosis was absent. On the other hand, H-FLACs exhibited tightly packed, glandular structures lined by columnar cells (Figure 2A). The latter is characterized by the presence of clear cytoplasm, large vesicular nuclei, subnuclear vacuoles, prominent nucleoli, and frequent mitotic. As opposed to L-FLACs, in all examined H-FLACs cases, morules were uncommon, while multifocal necrosis was quite frequent (Figure 2B). Moreover, miscellaneous histological components were observed in four out of five H-FLACs.
Three of the mixed cases identified a combination of H-FLAC and a conventional-type adenocarcinoma, with acinar, micropapillary and solid patterns observed. One of the mixed cases exhibited evidence of the presence of large cell neuroendocrine carcinoma. Lymphatic and small vein invasion was observed in two cases, and lymph node metastasis was detected in three cases.
Immunohistochemical findings
L-FLACs and H-FLACs produced different immunohistochemical findings (Figures 1,2). With respect to β-catenin expression, this protein was nuclear and cytoplasmic in the L-FLACs (3/3) (Figure 1B) but membranous in the H-FLACs (5/5) (Figure 2C). All L-FLAC cases and one H-FLAC case identified positive staining for TTF-1 (Figures 1C,2D). All 5 H-FLACs were immunohistochemically characterized by overexpression of the p53 protein (Figure 2E), whereas all three L-FLACs were negative for p53 (Figure 1D). Two H-FLAC cases were positive for AFP (Figure 2F), whereas all L-FLACs were negative for AFP. All H-FLAC cases were positive for membranous EGFR, whereas only one L-FLAC was positive for EGFR. As for the neuroendocrine markers, all three L-FLAC cases were positive for neuroendocrine markers (chromogranin A and synaptophysin). Four of the five H-FLAC cases were positive for neuroendocrine markers. In the H-FLAC case mixed with large cell neuroendocrine carcinoma (NEC) component, the large cell NEC component was strongly positive for neuroendocrine markers, while the other components were weakly positive. Immunostaining for Her-2 and ALK-D5F3 produced negative findings for all L-FLACs and H-FLACs.
Molecular characteristics
Mutation analyses of multiple genes were performed in all cases. The L858R point mutation in EGFR was detected in one H-FLAC case (case 6). The T790M EGFR mutation was observed in one L-FLAC case (case 4). The remaining cases had wild-type EGFR. No mutations were found in KRAS, PIK3CA or BRAF in any of the cases.
Discussion
FLAC is a rare lung adenocarcinoma subtype that is reminiscent of a developing fetal lung. With its unique clinicopathological and molecular characteristics, L-FLACs has been commonly perceived as a distinct variant of lung cancer, and was termed well-differentiated fetal adenocarcinoma in the WHO classification published in 2004. On the contrary, few reports on H-FLACs were published (2,4,10), and the majority of existing literatures were from the same country, making it a less well recognized lesion. In the present study, we summarized the opinions from the latest version of WHO classification [2015], and adopted stricter enrollment criteria for case selection in a large, consecutive series, which required the presence of at least 50% fetal morphology (6) to provide a cleaner vision of the demographic, clinical, morphological, and immunohistochemical features of H-FLAC and L-FLACs.
As a rare disease, the knowledge to the epidemiology of FLAC was far from adequate. Presently, there’s only one study reporting the presence of 17 H-FLACs in over four thousand lung cancer cases, and the prevalence of H-FLAC in primary lung cancer was assumed to be approximately 0.4% (4). A Portuguese study mentioned the estimated prevalence of FLAC in all primary lung tumors to be 0.5%, although the origin of the data was not specified (5). Herein, we reported that the prevalence of H-FLAC and L-FLAC to be 0.54% and 0.32% in Chinese patients with primary lung adenocarcinoma. To the best of our knowledge, this is the first report on the precise prevalence of H-FLAC and L-FLAC in a large consecutive series of Chinese lung adenocarcinoma patients.
L-FLACs and H-FLACs exhibited significant differences regarding clinicopathological characteristics and biological behavior. Our results demonstrated that H-FLAC tended to occur more frequently in elderly men with a history of heavy smoking but that L-FLAC was typically found in younger patients; these findings are consistent with the results of prior reports (2,4,7,10). Additionally, H-FLAC was often at an advanced stage at presentation, with a poor prognosis; in contrast, L-FLAC, was predominantly at stage I, with favorable outcomes (2,4).
Histologically, both prior reports and our findings indicated that H-FLAC histology is commonly combined with other histologies (4,10). Morita et al. reported that H-FLACs were invariably admixed with components of other lung cancer types. Thus, H-FLACs often exhibited a certain degree of morphological heterogeneity and various growth patterns (4). Recent studies have observed that these admixed elements may be conventional-type adenocarcinomas (such as lepidic, papillary, acinar, and micropapillary adenocarcinomas), large cell neuroendocrine carcinomas or enteric adenocarcinomas; they may exhibit solid-clear cell patterns (10). While, on the other hand, the histological pattern of L-FLAC tends to be pure.
In addition to differences in clinicopathological features, L-FLACs and H-FLACs exhibit distinctive immunophenotypic and molecular biological characteristics. Sekine et al. and Nakatani et al. have suggested that Wnt signaling component up-regulation, such as β-catenin, is important for L-FLACs and biphasic pulmonary blastomas but not H-FLACs (11,12). Thus, L-FLACs appear to be driven by mutations in the β-catenin gene; immunohistochemical analyses have demonstrated aberrant nuclear and cytoplasmic staining of β-catenin in the epithelial cells of L-FLACs (11,13). Our results demonstrated that β-catenin was observed to be nuclear and cytoplasmic in L-FLACs but membranous in H-FLACs; these results further support previous findings. In addition, p53 mutations have been identified in a range of cancers, including breast cancer, colon cancer, pancreas, ovary, bladder and oesophagus (14). Cornianu et al. reported that high p53 expression was correlated with poor differentiation, smoking history, advanced stage and low survival rate in patients with lung adenocarcinoma (15). In our study, all H-FLACs exhibited high p53 protein expression, whereas the L-FLACs were negative for p53; these findings also support the results of prior studies (2,15). AFP staining was positive in 2 cases (2/5). This observation is consistent with prior studies that have found AFP expression in 29–47% of H-FLACs (4).
All fetal adenocarcinoma cases in this study were negative for Her-2 and ALK D5F3. Also, no mutations were found in KRAS, PIK3CA or BRAF. EGFR mutations were detected in 2 cases (1 L-FLAC case and 1 H-FLAC case). In our previous study, in Chinese patients, EGFR mutations were observed in 51.2% of lung adenocarcinoma cases (16). This finding suggests that fetal adenocarcinomas may have distinctive molecular features.
Presently, the criteria and definition for H-FLACs varied in different studies, and it is still controversial as to whether it is a distinct variant of lung cancer or merely a morphological pattern with specific clinicopathological characteristics (2,4,10). Our results showed that, all 5 H-FLACs were immunohistochemically characterized by overexpression of the p53 protein, with rather similar clinicopathological and molecular features. Given the above observation, we tend to consider H-FLAC a unique histological subtype. As the sample size of the present study was rather small, studies based on the latest WHO classification with larger sample size are anticipated to provide a further insight to these tumors.
In the present study, L-FLAC is usually discovered at stage I, and tended to confer lower death rates. While on the contrary, H-FLAC is often discovered in advanced stages, and contributed to higher death rates. As tumor staging is a definitive prognosticator for lung cancer, it is difficult to tell whether the observation that H-FLAC and L-FLAC conferred distinct outcomes was a result of confounding by difference in distribution of the staging. Due to the relatively limited FLAC cases in the present study, we were unable to conduct further statistical analysis, thus in-depth investigation using a larger FLAC case series is desired to reveal the prognostic significance of the diagnosis of H-FLAC and L-FLAC.
In summary, L-FLACs and H-FLACs exhibit distinctive clinicopathological, immunophenotypical and molecular features with potential prognostic value. No difference was observed regarding the major driver gene mutations known in lung adenocarcinoma and H-FLAC.
Acknowledgements
None.
Footnote
Conflicts of Interests: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Peking Union Medical College Hospital with the approval number S-424, and informed consent was obtained for all cases.
References
- Kradin RL, Young RH, Dickersin GR, et al. Pulmonary blastoma with argyrophil cells and lacking sarcomatous features (pulmonary endodermal tumor resembling fetal lung). Am J Surg Pathol 1982;6:165-72. [Crossref] [PubMed]
- Nakatani Y, Kitamura H, Inayama Y, et al. Pulmonary adenocarcinomas of the fetal lung type: a clinicopathologic study indicating differences in histology, epidemiology, and natural history of low-grade and high-grade forms. Am J Surg Pathol 1998;22:399-411. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. [Crossref] [PubMed]
- Morita S, Yoshida A, Goto A, et al. High-grade lung adenocarcinoma with fetal lung-like morphology: clinicopathologic, immunohistochemical, and molecular analyses of 17 cases. Am J Surg Pathol 2013;37:924-32. [Crossref] [PubMed]
- El Ouazzani H, Jniene A, Bouchikh M, et al. Well-differentiated fetal adenocarcinoma: a very uncommon malignant lung tumor. Rev Port Pneumol 2012;18:39-41. [Crossref] [PubMed]
- WD Travis EB, AP Burke, A Marx, AG Nicholson. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer; 2015.
- Sato S, Koike T, Yamato Y, et al. Resected well-differentiated fetal pulmonary adenocarcinoma and summary of 25 cases reported in Japan. Jpn J Thorac Cardiovasc Surg 2006;54:539-42. [Crossref] [PubMed]
- Patnayak R, Jena A, Rukmangadha N, et al. Well-differentiated fetal adenocarcinoma of the lung in an adult male: report of an unusual tumor with a brief review of literature. J Cancer Res Ther 2014;10:419-21. [Crossref] [PubMed]
- Kanno R, Yamaura T, Higuchi M, et al. Well-differentiated fetal adenocarcinoma of the lung in a 20-year-old woman. Fukushima J Med Sci 2013;59:89-92. [Crossref] [PubMed]
- Suzuki M, Yazawa T, Ota S, et al. High-grade fetal adenocarcinoma of the lung is a tumour with a fetal phenotype that shows diverse differentiation, including high-grade neuroendocrine carcinoma: a clinicopathological, immunohistochemical and mutational study of 20 cases. Histopathology 2015;67:806-16. [Crossref] [PubMed]
- Sekine S, Shibata T, Matsuno Y, et al. Beta-catenin mutations in pulmonary blastomas: association with morule formation. J Pathol 2003;200:214-21. [Crossref] [PubMed]
- Nakatani Y, Masudo K, Miyagi Y, et al. Aberrant nuclear localization and gene mutation of beta-catenin in low-grade adenocarcinoma of fetal lung type: up-regulation of the Wnt signaling pathway may be a common denominator for the development of tumors that form morules. Mod Pathol 2002;15:617-24. [Crossref] [PubMed]
- Nakatani Y, Miyagi Y, Takemura T, et al. Aberrant nuclear/cytoplasmic localization and gene mutation of beta-catenin in classic pulmonary blastoma: beta-catenin immunostaining is useful for distinguishing between classic pulmonary blastoma and a blastomatoid variant of carcinosarcoma. Am J Surg Pathol 2004;28:921-7. [Crossref] [PubMed]
- Meek DW. Regulation of the p53 response and its relationship to cancer. Biochem J 2015;469:325-46. [Crossref] [PubMed]
- Cornianu M, Tudose N, Potencz E, et al. Expression and significance of tumoral suppressor p53 gene in lung adenocarcinomas. Rom J Morphol Embryol 1996;42:73-82. [PubMed]
- Zhang J, Liang Z, Gao J, et al. Pulmonary adenocarcinoma with a micropapillary pattern: a clinicopathological, immunophenotypic and molecular analysis. Histopathology 2011;59:1204-14. [Crossref] [PubMed]


