Optical coherence tomography in imaging of peripheral pulmonary arteries
Introduction
Optical coherence tomography (OCT) is an emerging technique capable of producing high-resolution optical images; it has been widely used in ophthalmic and coronary artery imaging (1,2). However, its application for the imaging of pulmonary arteries is still in its infancy. In recent years, OCT has been used to detect peripheral pulmonary thrombi (3) and identify idiopathic and thromboembolic pulmonary hypertension (4,5). Intravascular OCT requires a blood-free environment, which can be achieved by proximal vessel occlusion with an occlusion balloon catheter (OBC) and simultaneous flushing of the vessel lumen with a replacement fluid. The inflation pressure in the occlusion balloon and the flushing speed used are key parameters for OCT, and the desired occlusive effect may not be obtained if these are set too low. However, a high inflation pressure will cause vessel wall injury, dissection or tear if it exceeds the vascular tolerance. Similarly, a high flushing rate can cause vascular damage, myocardial ischemia, hypoxia, malignant arrhythmia and other consequences. The OBC designed by LightLab is based on coronary structure and blood-flow characteristics, and requires an inflation pressure of ≤1 atm (101.3 kPa) and a flushing rate of ≤1.0 mL/s. For coronary imaging, the recommended inflation pressure for the OBC ranges from 0.3 to 0.5 atm and the flushing rate is <0.5 mL/s (6,7). The coronary and pulmonary arteries differ significantly from each other in terms of vessel-wall structure and blood pressure. Therefore, it is often difficult to acquire suitable images of the pulmonary arteries by using the recommended parameters based on the early clinical practice guidelines for OCT. No specific operational procedure and parameter settings have been determined for OCT of the pulmonary arteries. To acquire optimal OCT images of the peripheral pulmonary arteries without causing vascular injury, we modified the conventional OCT method used for the coronary arteries. We used the percutaneous transluminal coronary angioplasty procedure, with a contrast agent as an indicator of balloon inflation, as our reference. We inflated the occlusion balloon under X-ray monitoring to ensure that the outer diameter of the balloon did not exceed the inner diameter of the blood vessels, and we simultaneously increased the flushing rate of the replacement fluid to perform OCT imaging of the peripheral pulmonary arteries. To determine which imaging method was better for pulmonary artery imaging, we compared the percentage of optimal OCT images and operative complications between the conventional OCT imaging method (COI) and the improved pulmonary artery imaging method (IPI).
Methods
Subjects
This study was approved by the Human Research Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University and Guangzhou Institute of Respiratory Diseases (approval number: GZGYHR-2009-04-20-02). Before the imaging, the participants and their families were explained, and they fully understood, the necessity and possible risks of such imaging. All participants provided written informed consent and consent for OCT imaging before enrollment in the study.
We enrolled patients who underwent OCT imaging of the peripheral pulmonary arteries for diagnostic purposes in the First Affiliated Hospital of Guangzhou Medical University and Guangzhou Institute of Respiratory Diseases between September 2009 and September 2010. Patients required OCT imaging of the peripheral pulmonary arteries if a peripheral pulmonary embolism or idiopathic pulmonary hypertension was highly suspected but there was insufficient evidence for a definite diagnosis. The exclusion criteria were as follows: (I) age <14 years; (II) chronic renal insufficiency (serum creatinine >133 µmol/L or >1.8 mg/dL); and (III) allergy to contrast agent or pregnancy or lactation.
Equipment
Angiography equipment
INNO-VA2000, a fully digital X-ray vascular angiography instrument (GE, USA). Contrast enhancement was performed using a 5 or 6 Fr arterial sheath (Terumo, Japan); guiding catheter, multi-purpose imaging tube (MP, Cordis Corporation, Miami Lakes, FL); pigtail angiographic catheter (MP, Cordis Corporation); long guidewire with hydrophilic coating (outer diameter, 0.035 inch; length, 260 cm; Amplatz, USA); soft-top guidewire (outer diameter, 0.014 inch; length, 191 cm; PILOT series/CROSS series/BMW ATW, USA).
OCT equipment and main accessories
OCT imager M2 series intravascular imaging system (LightLab Imaging Inc., Westford, MA, USA) consisting of two monitors, two storage drawers, mouse, keyboard and connected components of probes. The accessories included an OCT imaging guidewire (outer diameter, 0.014 inch; length, 190 cm; ImageWireTM, LightLab Imaging Inc.), a HeliosTM OBC (LightLab Image Inc.) and a low-pressure balloon pressurization pump (Indeflator LID-1, GoodTec).
Materials
Heparin sodium (Tianjin Biochem Pharmaceutical Co. Ltd., Tianjin, China) at a dose of 60–100 U/kg for systemic anticoagulation, 2% lidocaine hydrochloride (Treechem BioTech Co. Ltd., Shanghai, China) for local anesthesia, visipaque, a contrast agent (iodixanol injection, 270 mg/mL; GE) at 100 mL/bottle (the specific amount was determined during operation, and the total amount for each operation was <200 mL), sterile saline (Chongqing Daxin Pharmaceutical Co. Ltd., Chongqing, China) to clean the catheters and guidewires and lactated Ringer’s solution as a replacement liquid (Qingzhou Yao Wang Pharmaceutical Co. Ltd., Qingzhou, China).
Methods
Relationship between outer diameter of OBC and inflation pressure
Currently, there are no detailed data on the relationship between inflation pressure and the outer diameter of the OBC. To determine this relationship, we measured the outer diameter of the OBC in vitro at various inflation pressures and plotted a curve of the observed values. Three new OBCs were randomly selected. A 5-ml syringe was connected to their proximal interfaces, and air was withdrawn from the lumen and balloon of the OBC. A low-pressure inflation pump was connected, and the balloon was inflated with a dilute contrast agent (1:1). The outer diameter of the balloon was measured with vernier calipers, and the data was recorded by two observers. The diameter at each pressure gradient was measured three times, and the average diameter was used. The plot of the pressure and outer diameter was drawn using Excel software.
Imaging procedure
Target vessels were selected for pulmonary angiography. The vessel contour was identified, and their inner diameter at the occlusion site was measured. The vessels were then imaged using the COI and IPI. Angiography was repeated after the procedure to determine if any vascular injury had occurred. Vascular dissection, intimal tear, full-thickness tear and any other injuries, if present, were recorded. Conservative management with observation or balloon occlusion was adopted depending on the severity of the injury.
Procedure of COI
The inflation pressure of the balloon was 0.3–0.5 atm, and the flushing rate of Ringer’s lactate was 0.3–0.5 mL/s. The specific pressure and speed were determined by the inner diameter of the vessels to be imaged, according to the recommended imaging parameters given in the manufacturer’s instructions for the M2 OCT system (7).
Procedure of IPI
A contrast agent diluted in saline solution (1:1 dilution) was used as an indicator of balloon inflation. Under X-ray monitoring, the occlusion balloon was inflated using a low-pressure pump. The initial inflation pressure was 0.3 atm, and this was increased by 0.01 atm/s, until optimal OCT images were acquired or the outer diameter of the balloon equaled the inner diameter of the vessel. Meanwhile balloon inflating, we performed flushing with lactated Ringer’s solution at an initial rate of 0.3 mL/s. If the images were unclear, the injection rate was increased by 0.1 mL/s until the maximum rate of 1.0 mL/s was reached or until optimal images were acquired (Figure 1).
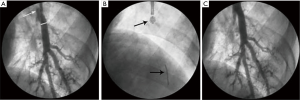
Observed indicators
The percentage of optimal OCT images was compared between the COI and IPI groups. The occlusion balloon pressure, flushing rate of replacement fluid and amount of replacement fluid required to produce optimal images were recorded. Related complications (including changes in electrocardiographic parameters, blood pressure and oxygen saturation), appropriate treatment and outcomes were also recorded. Complications, such as injury, dissection and tearing of the imaged vessels, were identified using repeat angiography after OCT imaging.
The criteria for optimal OCT images were as follows: (I) visualization of ≥285° of the vessel-wall perimeter; (II) uniform optical density across the lumen, allowing analysis of intraluminal lesions; and (III) images that were clear enough to show the three-tier structure of the vessel wall.
Statistical methods
The SPSS 12.0 statistical software was used for statistical analysis. Measurement data were expressed as mean and standard deviation. The χ2 test was used to compare percentages. The t-test was used to compare means between the two groups. Differences between the two groups were considered statistically significant at P values of <0.05.
Results
Patient information
Eight patients successfully underwent OCT imaging of the peripheral pulmonary
arteries with the COI and IPI. Their clinical data are shown in Table 1.
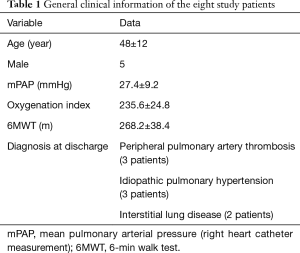
Full table
Relationship between outer balloon diameter and inflation pressure
The in vitro tests showed that the outer balloon diameter increased with increasing inflation pressure. The inflation pressure was increased in 0.01-atm increments from 0.1 to 1 atm. At inflation pressures of 0.1–0.5 atm, the outer balloon diameter increased significantly, by approximately 0.4 mm for each increment in pressure. At 0.6–0.8 atm, the diameter increased by 0.3 mm/increment, and at 0.9–1.0 atm, it increased by 0.2 mm/increment. To prevent vascular injury, the maximum pressure of the low-pressure pump was set at ≤1.0 atm. The curve representing the relationship between the outer balloon diameter and inflation pressure is presented in Figure 2.
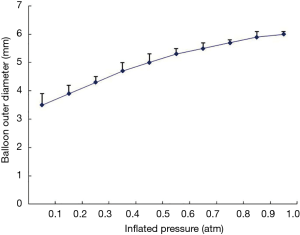
Parameters for OCT imaging of peripheral pulmonary arteries
We selected 23 peripheral pulmonary arteries in eight patients for imaging (average, 2.9±1.1 vessels per patient). OCT imaging was performed in 33 vessel segments; single-segment imaging was performed in 13 vessels, and double-segment imaging was performed in 10 vessels (Figure 3). The luminal diameters of the vessel segments at the occlusion site were 3.9–6.7 mm; 22 segments (67%) had inner diameters >5 mm. Optimal OCT images were acquired in only eight vessel segments in the COI group (24%) and 29 segments (88%) in the IPI group (P<0.01; Figure 4). The mean inflation pressure and mean flushing rate in the COI group were significantly lower than those in the IPI group (0.43±0.08 vs. 0.62±0.15 atm and 0.42±0.06 vs. 0.82±0.10 mL/s; both P<0.01). The amount of replacement fluid used for each vessel segment was lower in the COI group than in the IPI group (12.6±5.3 vs. 25.4±10.1 mL, P<0.01; Table 2).
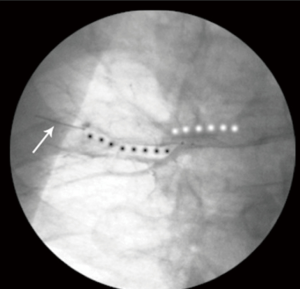


Full table
Complications
During OCT imaging, two patients in the COI group developed decreased transcutaneous blood oxygen saturation (SaO2; mean decrease, 8.4%±3.6%) after the occlusion of three vessel segments (9%, 3/33). One of these patients, who showed only a slight decrease, required no treatment. In the other patient, the SaO2 reverted to the pre-imaging level after approximately 10 min of low-flow oxygen therapy. No severe arrhythmia, ST-T changes, premature ventricular contractions, ventricular tachycardia or ventricular fibrillation occurred in the COI group.
In the IPI group, five patients developed decreased SaO2 (mean decrease, 13.1%±5.5%) after the occlusion of 10 vessel segments (30%, 10/33). One patient, who had only a slight decrease, required no treatment; in the other four patients, the SaO2 reverted to the pre-procedural levels with low-flow oxygen therapy administered after the procedure. The mean inner diameter (at the occlusion site) of the 13 vessel segments associated with decreased SaO2 was larger than the mean inner diameter of all 33 vessel segments examined (6.1±0.3 vs. 5.8±0.6 mm, P<0.05). In the IPI group, two patients aged >55 years (25%, 2/8) developed ST depression during OCT imaging (approximately 0.2 mv for about 40 s in one patient and approximately 0.1 mv for about 60 s in the other patient). Neither patient complained of chest tightness or pain. Moreover, in both patients, the ST-T ischemic changes disappeared when normal blood flow was restored after the imaging. Three patients (38%, 3/8) in the IPI group experienced occasional premature ventricular contractions during flushing with replacement fluid.
All patients no had sustained severe hypoxemia or required noninvasive or invasive mechanical ventilation treatment and no patients experienced chest pain or hemoptysis during the procedure. No severe complications, such as vascular injury, dissection or tear were observed during the OCT imaging. No premature ventricular contractions were observed after the imaging and no hemoptysis or shock was observed among any patient within 48 h after the imaging (Table 2).
Discussion
With the advent of intravascular OCT imaging, methods for blood clearance during vascular imaging have been continually improved. In the early stages, direct flushing with a contrast (8) or dextran 40 (9) were used; this method was simple but limited the imaging time and was likely to result in artifacts. With advancements in technology, the OBC (inner diameter, 0.48 mm) developed by LightLab has been used to advance OCT imaging guidewires and inject replacement fluid. This OBC can not only block proximal blood flow but also allows flushing with replacement fluid. The catheter is equipped with a compliant balloon, which arrests proximal blood flow upon inflation. In 2008, Yamaguchi et al. (10) conducted a multi-center study on the safety of the OBC during intracoronary OCT imaging and found that 97.3% optimal images were obtained was among 76 patients, at occlusion pressures of 0.4±0.1 atm and flushing rates of 0.6±0.4 mL/s. During the procedure, some patients experienced chest tightness, ST-T ischemic changes, bradycardia or tachycardia, but all of them recovered after the procedure. No vessel dissection, intravascular thrombosis, embolism, malignant arrhythmia, myocardial infarction or deaths occurred. These results demonstrated that intracoronary OCT imaging with an OBC was a safe procedure.
Coronary arteries gradually become thinner from their proximal to distal ends; their maximum inner diameter at the proximal end is approximately 4–5 mm (11). Our results about the relationship between inflation pressure and outer diameter of the occlusion balloon indicate that balloon inflation pressures of 0.3–0.5 atm can completely arrest proximal blood flow in these arteries. Yamaguchi et al. (10) found that the mean occlusion balloon pressure for intracoronary OCT imaging was 0.4±0.1 atm, whereas Chen (12) found that this pressure was 0.51±0.07 atm.
Vessel-wall structure and pressure significantly differ between the pulmonary and coronary arteries. Among the 29 vessel segments for which optimal OCT images were obtained in the IPI group, the inner (luminal) diameter at the occlusion site was 3.9–6.7 mm, and 67% vessels had luminal diameters >5 mm. The mean balloon inflation pressure in the IPI group was significantly higher than the pressure used for coronary imaging in previous studies [0.62±0.15 vs. 0.4±0.1 atm (10) or 0.51±0.07 atm (12)]. Consequently, optimal OCT images were acquired for only 24% of the imaged vessel segments in the COI group, in which an inflation pressure of 0.3–0.5 atm was used, as recommended in the manufacturer’s instructions. In contrast, optimal images were acquired for 88% of the imaged vessel segments in the IPI group, in which inflation pressures was based on the findings of our in vitro tests. The difference between the groups was significant. The prevalence of procedure-related complications increased with increasing inflation pressures and flushing speeds. In this study, decrease in SaO2 was a common complication. The mean internal diameter at the occlusion site of the 13 vessel segments that were associated with decreased SaO2 during imaging was greater than the mean internal diameter at the occlusion site of all 33 segments examined in this study. This indicates that decreased SaO2 is more likely to occur during the occlusion of large-caliber vessels. A possible explanation for this finding is that decreased SaO2 resulted from a ventilation-perfusion mismatch in the area that was predominantly supplied by the blood vessel being imaged, upon proximal occlusion of large-caliber vessels. The decreased SaO2 gradually recovered after oxygen therapy to elevate alveolar oxygen concentration or after relief of the occlusion at the end of imaging. No patient presented with persistent hypoxemia or required noninvasive or invasive mechanical ventilation during the OCT procedure.
Our results showed that the percentage of optimal OCT images was significantly increased by inflating the occlusion balloon with a contrast agent as the indicator and only gradually increasing the flushing speed. We found that optimal OCT images could be obtained for nearly 90% of the peripheral pulmonary arteries imaged with the common M2 OCT system. The mean balloon inflation pressure and mean flushing rate used to obtain this result were 0.62±0.15 atm and 0.82±0.10 mL/s, respectively. With increasing inflation pressures and flushing rates, the incidence of decreased SaO2 and ischemic ST-T changes also increased. However, upon oxygen inhalation or blood-flow restoration, both the SaO2 and ST-T changes quickly recovered without any serious complications. Patients with hypoxemia should be administered appropriate concentrations of oxygen prior to the imaging procedure in order to increase the body oxygen reserves and reduce complications. With advances in technology, new-generation OCT instrument non-occlusion technique has been developed. However, this new instrument is too expensive to be used in many hospitals in developing countries. Therefore, the M2 OCT system will still play an important role. Peripheral pulmonary vascular disorders such as idiopathic pulmonary artery hypertension and distal pulmonary thromboembolism are being increasingly researched. OCT is more suitable for imaging peripheral pulmonary vessels because of some unique advantages such as high-resolution images, real-time imaging and consecutive cross-sectional images. Therefore, it is necessary to explore the imaging method of OCT in peripheral pulmonary vasculature.
We consider the IPI method is safe and effective for OCT imaging in peripheral pulmonary artery. However, the results should be confirmed in large-scale, multi-center studies to overcome the limitations of the small sample size used in the present study.
Acknowledgements
Funding: This study is funded by the Natural Science Fund Project of Guangdong province (No. 2014A030310219).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Human Research Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University and Guangzhou Institute of Respiratory Diseases (No. GZGYHR-2009-04-20-02) and written informed consent was obtained from all patients.
References
- Jang IK, Bouma BE, Kang DH, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol 2002;39:604-9. [Crossref] [PubMed]
- Garcia-Garcia JG, Ruiz-Moreno JM, Holm K, et al. Macular dysfunction in drusen maculopathy assessed with multifocal electroretinogram and optical coherence tomography. Clin Ophthalmol 2013;7:1303-9. [Crossref] [PubMed]
- Hong C, Wang W, Zhong NS, et al. Using optical coherence tomography to detect peripheral pulmonary thrombi. Chin Med J (Engl) 2012;125:3171-4. [PubMed]
- Tatebe S, Fukumoto Y, Sugimura K, et al. Optical coherence tomography as a novel diagnostic tool for distal type chronic thromboembolic pulmonary hypertension. Circ J 2010;74:1742-4. [Crossref] [PubMed]
- Hou J, Qi H, Zhang M, et al. Pulmonary vascular changes in pulmonary hypertension: optical coherence tomography findings. Circ Cardiovasc Imaging 2010;3:344-5. [Crossref] [PubMed]
- Fercher AF, Drexler W, Hitzenberger CK, et al. Optical coherence tomography: principles and applications. Rep Prog Phys 2003;66:239-303. [Crossref]
- Optical coherence tomography instruction for use details. Available online: luder-us.aspx/. Published June 13, 2009. Accessed November 7, 2009.
- Prati F, Cera M, Ramazzotti V, et al. Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios. EuroIntervention 2007;3:365-70. [Crossref] [PubMed]
- Kataiwa H, Tanaka A, Kitabata H, et al. Safety and usefulness of non-occlusion image acquisition technique for optical coherence tomography. Circ J 2008;72:1536-7. [Crossref] [PubMed]
- Yamaguchi T, Terashima M, Akasaka T, et al. Safety and feasibility of an intravascular optical coherence tomography image wire system in the clinical setting. Am J Cardiol 2008;101:562-7. [Crossref] [PubMed]
- Chen BX. The factors impact on the imaging of optical coherence tomography. In: Chen BX, Jang IK, Akasaka T, et al. editors. Intracoronary imaging with optical coherence tomography. Beijing: Peking University Medical Press, 2009:34.
- Chen BX. The method of optical coherence tomography. In: Chen BX, Jang IK, Akasaka T, et al. editors. Intracoronary imaging with optical coherence tomography. Beijing: Peking University Medical Press, 2009:30.


