Are acute coronary syndromes an ideal scenario for bioresorbable vascular scaffold implantation?
Introduction
New-generation drug eluting stents (DES) are the “gold-standard” treatment for coronary artery disease (CAD) and are indicated in all patient and lesion subsets (1). However, because of permanent vessel caging a certain risk of restenosis or stent thrombosis persists years after percutaneous coronary intervention (PCI) (2). Therefore vascular restoration therapy, with the introduction of bioresorbable vascular scaffolds (BRS) has been developed to overcome these drawbacks, representing one of the most interesting innovations in the evolving field of coronary interventional cardiology. The aim of this new technology is to provide initial mechanical vessel scaffolding with drug release analogue to metal stent, thereafter the progressive struts resorption will theoretically preserve the physiological integrity of coronary arteries, restore reactive vasomotion, and allow long-term positive vessel remodeling and further repeat revascularizations if needed (3).
Among the several BRS being developed, the Absorb (BVS, Abbott Vascular, Santa Clara, CA, USA) has been most widely evaluated as a commercially available BRS. Initial findings showed similar mid-term outcomes from comparison between Absorb BVS and permanent everolimus eluting stent (EES, Xience, Abbott Vascular, Santa Clara, CA, USA) in stable patients with single de-novo, non-complex target lesions (4-7). However, meta-analysis of randomized trial showed similar efficacy but an increased risk of scaffold thrombosis (ScT) and target vessel myocardial infarction (TV-MI) in patients treated with BVS compared to EES (8,9). Furthermore, long-term follow-up of trials comparing BVS versus a metallic EES did not demonstrate the non-inferiority of BVS and confirmed a higher rate of ScT in the BVS group (10-12). Due to these unfavorable long-term results the European regulatory authorizes in accordance with the company have recently restricted the use of the device in Europe only to centers participating in clinical registries.
Different factors seem to contribute to the outcomes after BVS implantation. Appropriate vessel sizing seemed of paramount importance, as well as the operator experience and the use of a specific implantation technique demonstrated a significant reduction in BVS failures (13,14). Also the lesions type and the patient clinical presentation appear to play an important role on BVS performance. It is known that the risk of stent thrombosis after PCI is increased in patients with acute coronary syndromes (ACS) on admission, particularly after ST-segment elevation myocardial infarction (STEMI) (15), even with metallic DES. Given the greater BVS strut thickness and a higher risk of malapposition due to undersizing, concerns had been raised on the BVS safety with regard to ScT, especially in the hypercoagulable setting of ACS.
Therefore we will discuss in this review about the results from registries and randomized controlled trials evaluating the clinical outcome of BVS in ACS patients.
Rational for BVS use in ACS patients
The term ACS refers to any group of clinical symptoms compatible with acute myocardial ischemia that includes unstable angina (UA), non-STEMI (NSTEMI), and STEMI (16). ACS are usually associated with rupture of an atherosclerotic plaque and partial or complete thrombosis of the infarct-related artery.
Initial trials assessing BVS safety mainly excluded patients presenting with ACS. However ACS patients could theoretically benefit more from the vascular restoration therapy compared to stable patients for several reasons. ACS patients are often young, with a long life expectancy. In this case, as the BVS resorption and the restoration of coronary vessel normality take at least 3–4 years to complete, the time of the whole process cannot be considered too long. Younger patients have also a lower incidence of previous myocardial infarction (MI), and of previous PCI with stent implantation that would preclude the recovery of the pristine vessel state. Moreover acute lesions are generally soft plaque easy to expand thus facilitating optimal BVS deployment. Furthermore in thrombus-rich plaques, the greater BVS struts thickness compared to metallic DES, might reduce the risk of distal embolization and the no reflow phenomenon, by entrapping more thrombotic material to the vessel wall (17). This could also counterbalance the need of a more aggressive post-dilatation that is generally not recommended in the acute setting. Finally, it has been shown that the soft necrotic core of STEMI patient lesions may interfere with vascular healing more after DES than after BVS implantation (18).
On the other hand, a higher rate of ScT has been described in patients receiving BVS compared to DES, both in early and in late phase after the implantation. Particularly ScT rate was significantly increased in those studies that had included a larger number of patients with ACS (19). It is not surprising as stent thrombosis represents an important issue in the ACS setting even with newer DES as it is potentially triggered by enhanced platelets aggregation. However the larger struts of the BVS might impact much more on shear stress compared to the thinner struts of current metallic DES accelerating thrombus formation in such a pro-thrombotic state as ACS.
Observational registries assessing BVS performance in ACS patients
Observational registries can offer a valid overview over BVS performance in the real-world practice.
Single-center registries
First real-world data on BVS use in ACS come from Kajiya et al. (20) that first reported a single-center series of 11 STEMI patients treated with primary PCI and BVS implantation. Procedural success was achieved in all patients and early outcomes showed favorable results. Only one patient died due to cardiogenic shock and no other adverse event occurred within 30 days. Wiebe et al. (21) also evaluated BVS in 25 ACS patients (median follow-up 137 days) showing a major adverse cardiac event (MACE) rate of 8.3% and 1 ScT. In the BVS STEMI (22) first (49 patients) procedural success was 97.9%. Optical coherence tomography (OCT) revealed a low malapposition rate with only seven scaffolds presenting >5% of malapposed struts. At the 30-day follow-up, the target lesion failure rate was 0% and no cases of cardiac death or ScT were observed. Kochman et al. (23) performed an OCT assessment of acute procedural results of BVS implantation in 23 STEMI patients, demonstrating a high strut apposition rate (>95%) immediately after implantation. In a median follow-up period of 229 (range, 199–248) days, 1 MI, caused by sub-acute ScT, occurred in a patient who discontinued dual antiplatelet therapy (DAPT). They also reported the results of a 12-month OCT follow-up (24), showing a decrease in the mean lumen area, but no significant change in the mean scaffold area. Significant decreases in the malapposed strut ratio (4.9±8.65% vs. 0.4±1.55%, P<0.001) and malapposition area (0.29±0.60 vs. 0.08±0.32 mm2, P=0.002) were also observed. Gori et al. (25) reported the 1-year clinical outcomes and OCT analysis of 133 ACS patients (38% STEMI). Post-dilation was performed in 11% of the cases while DAPT with ticagrelor end/or prasugrel was administered in 78% of BVS patients. Restenosis rate was 4% and in-segment late lumen loss was 0.19±0.45 mm. OCT showed a malapposition rate of 26%. Endothelium dependent and independent vasodilation was observed in 48% and 49% of scaffold segments. However a relatively high rate of ScT was reported: 3 (2.3%) definite and 1 (0.8%) probable ScT, interestingly all occurred in the first 6 months. Encouraging results at long-term follow-up (18-months) after BVS implantation were reported in the Expand Registry (26) that included 249 patients with complex lesions (16.1% UA and 43% NSTEMI). Post-dilation was performed in 53.3% of the cases. Definite ScT rate was 1.9% with no cases of early thrombosis observed.
Multi-center registries
Numerous multicenter registries included ACS patients. The POLAR-ACS (27) registry included 100 ACS patients and reported favorable 1-year clinical results with MACE rate of 3% and 1 case of ScT. The ASSURE Registry (28) (21.3% UA) reported a 5% MACE rate at 1 year, showing that a slight systematic oversizing of the BVS, followed by high pressure post-dilatation, is safe and effective. Also short-term results of the RAI registry (1,505 patients/1,969 lesions, 59% ACS) confirmed that when meticulously implanted (96.8% post-dilation rate) BVS-related events may be mitigated (29). In the ISAR-ABSORB registry (30) (39% ACS) at 12 months, the incidence of target-lesion revascularization (TLR) among these patients was 13.1%, whereas definite ScT occurred in 2.6% of patients. A sub-analysis of the GHOST-UE trial (31) (47.1% ACS) showed that ACS at presentation was an independent predictor of both device-oriented endpoints (DOCE) and patient-oriented endpoints (POCE). No differences were found in TLR rate but ScT was significantly higher in ACS vs. stable CAD patients (2.6% vs. 0.8%, P=0.006). However in this registry BVS post-dilation rate was quite low particularly in in ACS group (46%) and DAPT with either ticagrelor or prasugrel was used only in 35.2% ACS patients. Also in a pooled analysis of the BVS Expand and BVS STEMI registries (32) (351 patients, 72.6% ACS), post-dilation in ACS group was only 41.3%. Interestingly, acute angiographic outcomes were better in ACS than in non-ACS, and no differences were noticed in 1-year clinical outcomes. However, early ScT occurred only in ACS patients. Interesting OCT data (33) from 29 BVS (62%ACS) showed that ACS patients had reduced neointimal growth and increased percentage of uncovered struts compared to stable CAD patients at follow-up. Finally a meta-analysis of 45 trials showed no correlation between ScT and ACS as clinical presentation (34). The PRAGUE-19 (35), focused only on STEMI (117 patients), reported 97% success rate while two cases of early ScT (1.7%) and no late/very late ScT at 3 years follow-up.
Comparison between BVS and DES in ACS population
The Mainz ACS (36) registry compared 150 patients treated with BVS vs. a control group composed of 103 consecutive patients who underwent DES implantation in the same period and reported similarly favorable outcome. Similarly a comparison of BVS vs. EES in STEMI patients with a high rate of post-dilation and new potent antiplatelet agents showed favorable mid-term results (37). The BVS EXAMINATION (38,39) is the largest propensity score matching comparison of BVS (290 patients) against both EES and bare-metal stents in patients with STEMI (patients derived from the EXAMINATION Trial). One- and 2-year follow-up did not show any significant differences regarding DOCE between groups (P=0.678). The rate of definite thrombosis tended to be higher in the BVS group, as compared with the EES group (3.3% vs. 1.0%; P=0.081), with a numerically higher, although not statistically significant rate of definite/probable ScT (4.0% vs. 2.1%; P=0.221) as well as a higher overall rate of thrombosis (4.4% vs. 2.4%, P=0.245) in the BVS group. In the 18 months follow-up of the BVS STEMI first (40) propensity score matching comparison between 151 BVS patients and 151 EES patients, the MACE rate was higher in the BVS group (9.8% vs. 3.6%, P=0.02, and TLR was 5.7% vs. 1.3%, P=0.05). Interestingly optimal implantation technique progressively increased during the length of the study and the 30-day MACE rate in BVS patients without post-dilatation was 6.8%, and this was reduced to 3.6% in patients with post-dilatation. ScT occurred primarily in the acute phase. Of note, all BVS cases with acute ScT had no post-dilatation at the index procedure suggesting that the optimization of the implantation technique is of paramount importance even in the acute setting. Imori et al. also confirmed the importance of BVS post-dilation in the ACS setting (41). At 24 months follow-up a higher rate of MACE was observed in BVS compared to EES in consecutive ACS patients before and after propensity score matching. However, after sensitivity analysis, MACE rates in BRS patients with post-dilation were significantly lower than in those without post-dilation and comparable to EES patients (6.0% vs. 12.6% vs. 4.7%, P<0.001). ScT rates were only slightly lower in the BVS group with post-dilatation but were higher in both the BVS groups than in EES patients (2.0% vs. 2.6% vs. 1.2%, P=0.09).
Randomized trials assessing BVS performance in ACS patients
The initial randomized trials evaluating BVS performance compared to second generation DES mainly included simple lesions in stable patients. The first randomized trial including ACS patients in the comparison between BVS vs. EES was the EVERBIO II trial (42), an assessor-blinded study that enrolled 240 patients (39% presenting with ACS, 9.5% STEMI) randomly assigned BVS or EES (Promus Element; Boston Scientific, Marlborough, Massachusetts, USA) or biolimus-eluting stent (Biomatrix Flex, Biosensors Europe SA, Morges, Switzerland). The angiographic late lumen loss, primary endpoint at 9 months, as well as the patient and device-oriented endpoints did not differ between study groups. No definite stent thrombosis was reported at follow-up, while one possible ScT was reported in the BVS group.
The TROFI II trial (18) focused exclusively on STEMI (191 patients) undergoing primary PCI, randomly allocated 1:1 to treatment with the BVS or EES. Primary endpoint was the arterial healing score at 6 months follow-up assessed by OCT. A low HS indicates a favorable healing process without intraluminal luminal defect, malapposition or uncovered struts, etc., whereas a high HS reflects a poor healing process with remnant thrombus, uncovered and/or malapposed struts. Results showed a lower healing score in the BVS compared to EES group (1.74±2.39 vs. 2.80± 4.44; difference 21.06, 90% CI, 21.96–20.16; P non-inferiority =0.001), with a trend suggesting better healing process in the BVS group (P for superiority =0.053). Quantitative angiography showed a lower late lumen loss in the EES group, however the device-oriented composite endpoint was comparably low between groups (1.1% BVS vs. 0% EES). One case of definite sub-acute ScT occurred in the BVS group (1.1% vs. 0% EES; P= ns).
Recently the two years results of the AIDA trial (12) have been reported; a prospective, randomized (1:1), active-control, single-blinded, all-comer, non-inferiority trial that randomized patients to treatment with the BVS or EES. The trial included 1,845 patients with both simple and complex lesions. 54% of patients presented with ACS and 25% of them underwent primary PCI for acute myocardial infarction. The AIDA investigators performed an interim analysis of their data because of safety concerns. They early reported the 2 years results showing that target vessel failure, the study’s primary endpoint, occurred at comparable rates between BVS and EES (11.7% versus 10.7%, HR 1.12, 95% CI, 0.85–1.48). There was no difference in the risk of cardiac death between the two devices, but the risk of MI was significantly higher with BVS, including a higher risk of TV-MI (5.5% vs. 3.2%; P=0.04). Definite/probable ScT was significantly higher in BVS vs. EES (3.5% vs. 0.9%, HR 3.87; 95% CI, 1.78–8.42). The higher risk of ScT was evident in the sub-acute phase (>24 h to 30 days) as well as late and very late phases, including beyond 1 year. Contrarily to the ABSORB III 2-year results, the investigators did not find any relation between clinical outcomes and either the implantation technique (74% BVS post-dilation rate) or the diameter of the treated vessels or the presenting symptoms. However, among the patients in the scaffold group who had definite or probable device thrombosis, 19% had a residual diameter stenosis of 30% or greater; among the patients who did not have device thrombosis, 9% had a residual percent diameter stenosis of 30% or greater (P=0.05) highlighting the importance to obtain maximal BVS expansion at the end of the procedure.
Table 1 shows registries and clinical trial enrolling more than 100 BVS and including ACS patients.
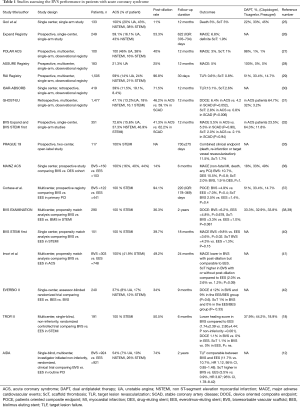
Full table
Evidence for other bioresorbable devices use in ACS population
Along to the Absorb BVS other two BRS received the CE mark: the novolimus-eluting DESolve (Elixir Medical Corporation, Sunnyvale, CA, USA) and the sirolimus-eluting absorbable metal scaffold Magmaris (Biotronik AG, Buelach, Switzerland). However, very limited data are available on those new BRS in ACS patients. The DESolve is a polymeric BRS that has different features compared to Absorb. In particular, it has a higher radial strength, a faster degradation process (that takes approximately 1 to 2 years), and it can be over-expanded up to 0.5 mm above the nominal diameter. In a bench test it has been showed that DESolve has the ability to tolerate even a higher over expansion during post-dilation without fracture (e.g., a 3.0 mm DESolve has been shown to did not fracture at diameters of 5.0 mm or less while a 3.0 mm Absorb did not fracture at diameters of 3.8 mm or less at 20 atm pressure) (43). Furthermore, it is able to self-correct for minor malapposition, which is particularly appealing in highly thrombotic lesions. In a propensity score matching comparison (44) between Absorb BVS vs. DESolve (approximately 50% of ACS and 85% of post-dilation rate in both groups) no relevant differences were reported in the clinical outcomes at 1 year, and ScT rate was 2.0% and 1.0% (P=0.529) in Absorb vs. DESolve respectively. The Magmaris is an absorbable magnesium scaffold coated with bioresorbable poly-l-lactide incorporating sirolimus, and it showed promising 1-year outcomes in stable patients (45). However to date no data are available on Magmaris use in ACS patients and the manufacturer contraindicate the use of this new BRS in this setting.
Conclusions
The introduction of the vascular reparative therapy with BRS represents one of the most interesting technological advances in the field of interventional cardiology. However current BRS present several drawbacks as high strut thickness and low radial strength, limiting the device navigability and performance. In addition, resorption time seems to be longer than originally predicted on the basis of animal studies. The first real-world experiences with BVS had raised concerns on the rate of device thrombosis and contrasting results have been reported on the BVS performance in ACS patients. However, based on the evidence discussed above, the adoption of a specific implantation technique (including the strongest DAPT regimen available) seems of paramount importance to reduce BVS failure even in the acute setting. The upcoming BVS STEMI STRATEGY-IT study (46) will provide further data to better understand the impact of a pre-specified BVS implantation strategy in STEMI patients undergoing primary PCI, while the ongoing trials such as the Compare Absorb (NCT02486068), will provide data derived from larger patient cohorts and direct comparison to metallic DES. The development of new BRS generations, with reduction in struts thickness together with the improvement in radial strength and the self-correction will probably represent an important step forward in solving current BRS limitations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- Nakamura D, Attizzani GF, Toma C, et al. Failure Mechanisms and Neoatherosclerosis Patterns in Very Late Drug-Eluting and Bare-Metal Stent Thrombosis. Circ Cardiovasc Interv 2016.9. [PubMed]
- Serruys PW, Garcia-Garcia HM, Onuma Y. From metallic cages to transient bioresorbable scaffolds: change in paradigm of coronary revascularization in the upcoming decade? Eur Heart J 2012;33:16-25b. [Crossref] [PubMed]
- Serruys PW, Chevalier B, Dudek D, et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2015;385:43-54. [Crossref] [PubMed]
- Ellis SG, Kereiakes DJ, Metzger DC, et al. ABSORB III Investigators. Everolimus-Eluting Bioresorbable Scaffolds for Coronary Artery Disease. N Engl J Med 2015;373:1905-15. [Crossref] [PubMed]
- Gao R, Yang Y, Han Y, et al. Bioresorbable Vascular Scaffolds Versus Metallic Stents in Patients With Coronary Artery Disease: ABSORB China Trial. J Am Coll Cardiol 2015;66:2298-309. [Crossref] [PubMed]
- Kimura T., Kozuma K, Tanabe K, et al. A randomized trial evaluatingeverolimus-eluting Absorb bioresorbable scaffolds versus everolimus-eluting metallic stents in patients with coronary artery disease: ABSORB Japan. Eur Heart J 2015;36:3332-42. [Crossref] [PubMed]
- Stone GW, Gao R, Kimura T, et al. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet 2016;387:1277-89. [Crossref] [PubMed]
- Cassese S, Byrne RA, Ndrepepa G, et al. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. Lancet 2016;387:537-44. [Crossref] [PubMed]
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016;388:2479-91. [Crossref] [PubMed]
- Onuma Y, Sotomi Y, Shiomi H. Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an everolimus eluting bioresorbable scaffold and an everolimus-eluting metallic stent: insights from the randomised ABSORB Japan trial. EuroIntervention 2016;12:1090-101. [Crossref] [PubMed]
- Wykrzykowska JJ, Kraak RP, Hofma SH, et al. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N Engl J Med 2017;376:2319-28. [Crossref] [PubMed]
- Puricel S, Cuculi F, Weissner M, et al. Bioresorbable Coronary Scaffold Thrombosis: Multicenter Comprehensive Analysis of Clinical Presentation, Mechanisms, and Predictors. J Am Coll Cardiol 2016;67:921-31. [Crossref] [PubMed]
- Ortega-Paz L, Capodanno D, Gori T, et al. Predilation, sizing and post-dilation scoring in patients undergoing everolimus-eluting bioresorbable scaffold implantation for prediction of cardiac adverse events: development and internal validation of the PSP score. EuroIntervention 2017;12:2110-7. [Crossref] [PubMed]
- Kukreja N, Onuma Y, Garcia-Garcia HM, et al. Interventional Cardiologists of the Thoraxcenter (2000 to 2005). The risk of stent thrombosis in patients with acute coronary syndromes treated with bare-metal and drug-eluting stents. JACC Cardiovasc Interv 2009;2:534-41. [Crossref] [PubMed]
- Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc 2009;84:917-38. [Crossref] [PubMed]
- Diletti R, van der Sijde J, Karanasos A, et al. Differential thrombotic prolapse burden in either bioresorbable vascular scaffolds or metallic stents implanted during acute myocardial infarction: The snowshoe effect: Insights from the maximal footprint analysis. Int J Cardiol 2016;220:802-8. [Crossref] [PubMed]
- Sabaté M, Windecker S, Iñiguez A, et al. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J 2016;37:229-40. [Crossref] [PubMed]
- Lipinski MJ, Escarcega RO, Baker NC, et al. Scaffold thrombosis after percutaneous coronary intervention with ABSORB bioresorbable vascular scaffold a systematic review and meta-analysis. JACC Cardiovasc Interv 2016;9:12-24. [Crossref] [PubMed]
- Kajiya T, Liang M, Sharma RK, et al. Everolimus-eluting bioresorbable vascular scaffold (BVS) implantation in patients with ST-segment elevation myocardial infarction (STEMI). EuroIntervention 2013;9:501-4. [Crossref] [PubMed]
- Wiebe J, Möllmann H, Most A, et al. Short-term outcome of patients with ST-segment elevation myocardial infarction (STEMI) treated with an everolimus-eluting bioresorbable vascular scaffold. Clin Res Cardiol 2014;103:141-8. [Crossref] [PubMed]
- Diletti R, Karanasos A, Muramatsu T, et al. Everolimuseluting bioresorbable vascular scaffolds for treatment of patients presenting with ST-segment elevation myocardial infarction: BVS STEMI first study. Eur Heart J 2014;35:777-86. [Crossref] [PubMed]
- Kochman J, Tomaniak M, Pietrasik A, et al. Bioresorbable everolimus-eluting vascular scaffold in patients with ST-segment elevation myocardial infarction: Optical coherence tomography evaluation and clinical outcomes. Cardiol J 2015;22:315-22. [Crossref] [PubMed]
- Kochman J, Tomaniak M, Kołtowski Ł, et al. A 12-month angiographic and optical coherence tomography follow-up after bioresorbable vascular scaffold implantation in patients with STsegment elevation myocardial infarction. Catheter Cardiovasc Interv 2015;86:E180-9. [Crossref] [PubMed]
- Gori T, Schulz E, Hink U, et al. Clinical, angiographic, functional, and imaging outcomes 12 months after implantation of drug-eluting bioresorbable vascular scaffolds in acute coronary syndromes. JACC Cardiovasc Interv 2015;8:770-7. [Crossref] [PubMed]
- Felix CM, Fam JM, Diletti R, et al. Mid- to long-term clinical outcomes of patients treated with the everolimus-eluting bioresorbable vascular scaffold: The BVS Expand Registry. JACC Cardiovasc Interv 2016;9:1652-63. [Crossref] [PubMed]
- Dudek D, Rzeszutko Ł, Zasada W, et al. Bioresorbable vascular scaffolds in patients with acute coronary syndromes: the POLAR ACS study. Pol Arch Med Wewn 2014;124:669-77. [PubMed]
- Wöhrle J, Naber C, Schmitz T, et al. Beyond the early stages: insights from the ASSURE registry on bioresorbable vascular scaffolds. EuroIntervention 2015;11:149-56. [Crossref] [PubMed]
- Cortese B, Ielasi A, Moscarella E, et al. Thirty-Day Outcomes After Unrestricted Implantation of Bioresorbable Vascular Scaffold (from the Prospective RAI Registry). Am J Cardiol 2017;119:1924-30. [Crossref] [PubMed]
- Hoppmann P, Kufner S, Cassese S, et al. Angiographic and clinical outcomes of patients treated with everolimus-eluting bioresorbable stents in routine clinical practice: Results of the ISAR-ABSORB registry. Catheter Cardiovasc Interv 2016;87:822-9. [Crossref] [PubMed]
- Schnorbus B, Wiebe J, Capodanno D, et al. 12 months outcomes after Bioresorbable Vascular Scaffold implantation in Patients with Acute Coronary Syndromes. Data from the European Multicentre GHOST-EU Extended Registry. EuroIntervention 2017. [Epub ahead of print].
- Felix CM, Onuma Y, Fam JM, et al. Are BVS suitable for ACS patients? Support from a large single center real live registry. Int J Cardiol 2016;218:89-97. [Crossref] [PubMed]
- Giblett JP, Brown AJ, Keevil H, et al. Implantation of bioresorbable vascular scaffolds following acute coronary syndrome is associated with reduced early neointimal growth and strut coverage. EuroIntervention 2016;12:724-33. [Crossref] [PubMed]
- Sotomi Y, Miyazaki Y, Collet C, et al. Does acute coronary syndrome impact on the incidenca of SCT after BVS? Eurointervention 2017;12:2025-7. [Crossref] [PubMed]
- Toušek P, Kočka V, Malý M, et al. Long-term follow-up after bioresorbable vascular scaffold implantation in STEMI patients: PRAGUE-19 study update. Eurointervention 2016;12:23-9. [Crossref] [PubMed]
- Gori T, Schulz E, Hink U, et al. Early outcome after implantation of Absorb bioresorbable drug-eluting scaffolds in patients with acute coronary syndromes. EuroIntervention 2014;9:1036-41. [Crossref] [PubMed]
- Cortese B, Ielasi A, Romagnoli E, et al. Clinical Comparison With Short-Term Follow-Up of Bioresorbable Vascular Scaffold Versus Everolimus-Eluting Stent in Primary Percutaneous Coronary Interventions. Am J Cardiol 2015;116:705-10. [Crossref] [PubMed]
- Brugaletta S, Gori T, Low AF, et al. Absorb bioresorbable vascular scaffold versus everolimus-eluting metallic stent in STsegment elevation myocardial infarction: 1-year results of a propensity score matching comparison: The BVS-EXAMINATION Study (bioresorbable vascular scaffold-a clinical evaluation of everolimus eluting coronary stents in the treatment of patients with ST-segment elevation myocardial infarction). JACC Cardiovasc Interv 2015;8:189-97. [Crossref] [PubMed]
- Brugaletta S, Gori T, Low AF, et al. ABSORB bioresorbable vascular scaffold vs. everolimuseluting metallic stent in ST-segment elevation myocardial infarction (BVS EXAMINATION study): 2-Year results from a propensity score matched comparison. Int J Cardiol 2016;214:483-4. [Crossref] [PubMed]
- Fam JM, Felix C, van Geuns RJ, et al. Initial experience with everolimus-eluting bioresorbable vascular scaffolds for treatment of patients presenting with acute myocardial infarction: a propensity-matched comparison to metallic drug eluting stents 18-month follow-up of the BVS STEMI first study. EuroIntervention 2016;12:30-7. [Crossref] [PubMed]
- Imori Y, D'Ascenzo F, Gori T, et al. Impact of postdilatation on performance of bioresorbable vascular scaffolds in patients with acute coronary syndrome compared with everolimus-eluting stents: A propensity score-matched analysis from a multicenter “real-world” registry. Cardiol J 2016;23:374-83. [Crossref] [PubMed]
- Puricel S, Arroyo D, Corpataux N, et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J Am Coll Cardiol 2015;65:791-801. [Crossref] [PubMed]
- Ormiston JA, Webber B, Ubod B, Darremont O, Webster MW. An independent bench comparison of two bioresorbable drug-eluting coronary scaffolds (Absorb and DESolve) with a durable metallic drug-eluting stent (ML8/Xpedition). EuroIntervention 2015;11:60-7. [Crossref] [PubMed]
- Wiebe J, Dörr O, Ilstad H, et al. Everolimus- Versus Novolimus-Eluting Bioresorbable Scaffolds for the Treatment of Coronary Artery Disease A Matched Comparison. JACC Cardiovasc Interv 2017;10:477-85. [Crossref] [PubMed]
- Haude M, Ince H, Abizaid A, et al. Sustained safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de novo coronary lesions: 12-month clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial. Eur Heart J 2016;37:2701-9. [Crossref] [PubMed]
- Ielasi A, Varricchio A, Campo G, et al. A prospective evaluation of a standardized strategy for the use of a polymeric everolimus-eluting bioresorbable scaffold in ST-segment elevation myocardial infarction: Rationale and design of the BVS STEMI STRATEGY-IT study. Catheter Cardiovasc Interv 2017;89:1129-38. [Crossref] [PubMed]


