Sputum mucin 1 is increased during the acute phase of chronic obstructive pulmonary disease exacerbation
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation which is usually progressive and associated with an enhanced chronic inflammatory response in the airways and lungs. It is generally considered that chronic inflammation is critical for COPD development, which damages the airway by influencing airways repair and remodeling (1). The acute exacerbation of COPD (AECOPD) is an acute event characterized by worsening respiratory symptoms which are beyond normal day-to-day variations, lead to changes in regular medication and might contribute to the overall severity in individual patients (2). COPD exacerbations can be precipitated by several factors. The most common causes are airway inflammations in respiratory tract induced by bacterial infections (3).
Mucin 1 (MUC1 in humans and Muc1 in non-human species) is a type I transmembrane (TE) glycoprotein ubiquitously expressed on the apical surface of epithelial cells in respiratory, gastrointestinal and reproductive tracts and on some immune cells surfaces (4). MUC1 contains three major domains, an extracellular (EC) domain, a TE domain and a cytoplasmic tail (CT) domain (5-7). Functions of MUC1 include protection and lubrication on epithelial surface. The CT domain of MUC1 mediates the cell signal transductions and cell adhesion. An indispensable anti-inflammatory role of Muc1 has been found in the airway during Pseudomonas aeruginosa and respiratory syncytial virus infection (8,9). Muc1 levels in bronchoalveolar lavage (BAL) fluid were increased during the acute phase of lung inflammation and positively correlated with TNF-α level increase (8,10) and neutrophil elastase levels upregulation (11). Chronic airway inflammation is one of characteristics of COPD. When compared with normal populations, higher MUC1 levels were found in both bronchiolar/alveolar epithelium and sputum from stable COPD patients (12). However, whether and how MUC1/Muc1 is changed during the exacerbation of COPD remains to be elucidated.
In this study, we determined the MUC1 levels in induced sputum from COPD patients in acute and remission phases of AECOPD. The association between MUC1 levels and COPD exacerbation was evaluated. To understand how sputum MUC1 levels are changed during AECOPD, a mouse model for acute airway inflammation was utilized and the patterns of Muc1 levels change in lung and BAL fluid were studied. As a result, we find that sputum MUC1 is increased in the acute phase of COPD and this increase is likely incurred from airway inflammation. In addition£¨sputum MUC1 is correlated with the severity of airway inflammation and lung function decline of COPD patients.
Methods
COPD subjects
The protocols of this study were approved by the ethic committee of The First Affiliated Hospital of Guangzhou Medical University. Seventy-eight patients were randomly recruited from inpatients with COPD exacerbation who were hospitalized in The First Affiliated Hospital of Guangzhou Medical University from April 2012 to August 2013. The detailed standards on defining AECOPD are described below. All patients were required to provide written consent before commencing the study, which was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University.
AECOPD define standards
AECOPD is defined when a patient has background COPD (1) and at least two major symptoms (increased dyspnea, increased sputum purulence or increased sputum production) or one major and one minor symptom (nasal discharge/congestion, wheeze, sore throat or cough) for at least two consecutive days (13-15), in combination with a post-bronchodilator FEV1/FVC ratio of 70%. The remission phase is judged when the symptoms are alleviated after standard medical treatment for 5 days at least. And the time point is defined according to following items: (I) significantly decreased radiographic infiltrations; (II) sputum turning thin and white with significantly less production; (III) at least one of the following signs accompanied: body temperature decreases to less than 37.5 °C, blood leukocyte count is below 10×109/L or is 2×109/L less than acute phase; and (IV) significant relief of dyspnea (16). The exclusion criteria include allergies, tumor, asthma and other respiratory diseases.
Collection of induced sputum and cell counting
Sputum was collected in both acute and remission phase from each patient and processed as described (17). Briefly, after pre-medication with 200 µg of inhaled salbutamol, subjects inhaled hypertonic (3%) saline solution delivered with an ultrasonic nebulizer for 15 to 30 min. Each collected sputum sample was immediately removed from contaminating saliva using forceps, weighted, and then lysed with phosphate-buffered saline solution (4 mL for every 1 g of sputum) containing 0.1% dithiothreitol (AMRESCO, USA) at 4 °C overnight. Then the sputum lysate was filtered through 48-µm nylon gauze and centrifuged (800 g for 10 min at 4 °C). The cell pellet was re-suspended in PBS, cytospined and stained with hematoxylin and eosin (H&E). The total and differential cell numbers were counted. The supernatants were collected and stored at −80 °C for other analysis.
Animals and LPS inhalation
Male C57BL/6 mice (6–8 weeks) were maintained under specific pathogen free (SPF) environmental conditions with unlimited access to food and water. All experimental protocols were approved by the Animal Care and Use Committees of Guangzhou Medical University. For intranasal LPS inhalation treatment, each mouse received LPS (7.5 µg in 50 µL PBS; L3024, Sigma) intranasally after being anesthetized with intraperitoneal pentobarbital injection (0.08 mg/g body weight). The mice were sacrificed at 12, 24, 48, 96 hours or 1 week after the LPS inhalation.
BAL fluid collection and differential cell counting
BAL fluid was obtained by BAL performed for three times with 0.8 mL sterile saline. The recovered lavage fluid was centrifuged at 3,000 rpm for 10 min. The cell-free supernatants were collected and stored at −80 °C for further analysis. The cell pellets were resuspended in saline, cytospined and stained using H&E for total and differential cell counting.
Lung histology
After LPS or saline inhalation, the right lung lobes were fixed in 4% paraformaldehyde and embedded in paraffin. The sections (4 µm) were stained with H&E.
ELISA
Mouse lung tissue were homogenized in RIPA lysis buffer containing 1% protease inhibitor cocktail (Sigma-Aldrich), 5 µM EDTA and 200 µM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (100 mg tissue in 1ml lysis buffer) and centrifuged to extract the total protein. The supernatants of the lung tissues homogenate were kept for ELISA. Direct ELISA was used to measure the protein levels of MUC1/Muc1 in induced sputum, mice lung tissues and BAL fluids. In order to avoid the interference of DTT or detergent in lysis buffer on ELISA, the supernatants of induced sputum (1:1,280), the mouse lung tissue lysates (1:2,000) or BAL fluid from mouse (1:640) were diluted in 0.05 mM bicarbonate buffer (pH 9.5) and used to coat the 96-well plates overnight at 4 °C. The coated plates were blocked with PBS-BSA and incubated with an anti-MUC1 CT domain antibody (clone EP1024Y, abcam) or an anti-MUC1 EC N-terminal domain antibody (GP1.4, Santa Cruz Biotechnology, 1:800 in blocking buffer) for detecting the CT or EC domains, respectively. MUC1/Muc1 concentration was determined from a pooled sample and expressed as arbitrary units. Measurements of the IL-8 and TNF-α level in induced sputum or BAL fluid were performed with ELISA kits (human IL-8 ELISA kit: 88-2087; human TNF-α ELISA kit: 88-7346; or mouse TNF-α ELISA kit: 88-7324; all from eBioscience) according to the manufacturer’s instructions. The cytokine concentrations were determined from standard curve of corresponding cytokine. Mouse keratinocyte derived chemokine (KC) levels were measured according to Choi’s methods (10). Briefly, the plate was coated with capture antibody (MAB-453, R&D system, 2 µg/mL in antibody dilution buffer (2.8% Triton X-100, 0.2% Tween-80 and 1% BSA in PBS) overnight at 4 °C. After washing and being blocked with blocking buffer (1% BSA and 1% sucrose in PBS), BAL fluid was added and incubated at room temperature for 2 hours. After washing, the biotinylated detection antibody (BAF-453, R&D system, 2 µg/mL in antibody dilution buffer) was added and incubated at room temperature for 2 hours. Finally, extravidin peroxidase (1:1,000 in PBS, Cat. E2886, Sigma) and TMB (tetramethylbenzidine) substrate reagent set (Cat. 555214, BD Biosciences Pharmingen) were used for detection. The concentration of KC was determined from a standard curve by using a KC standard (453-KC, R&D system).
Western blotting analysis
Lung tissues were homogenized in RIPA buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% Na deoxycholate, 2 mM EGTA, 2 mM EDTA, 0.1% SDS) containing protease inhibitor cocktail (1:100, Sigma). Lysates were sonicated, centrifuged at 12,000 g for 30 minutes at 4 °C and equalized the total protein concentrations with BCA protein quantification assay. Then equal total protein (40 µg) for each sample was loaded to SDS-PAGE gel and blotted with primary antibodies [anti-MUC1 (clone EP1024Y, Abcam) and anti-β-tubulin (Sigma)] at 4 °C overnight and corresponding secondary antibodies. Finally, the results were visualized with enhanced chemiluminescence (ECL).
Statistical analysis
All data were analyzed using a statistical software package (SPSS for Windows, version 16.0). Differences of cell counts in BAL fluid between treatment groups of mice were assessed using the Student’s t-test. One way ANOVA analysis was used to compare cytokine and Muc1 levels in BAL fluid between control and LPS treated groups at various time points. Comparison of data between acute and remission phase of patients was conducted using Mann Whitney U test. Values are presented as the mean ± SEM. “n” refers to the sample size (i.e., the number of patients or the animals received LPS or saline inhalation). Linear regression analysis was performed to study the independent effects of age, neutrophil counts and pro-inflammatory cytokines in sputum and lung function parameters of COPD patients on MUC1 levels. *P<0.05 and **P<0.01 were considered different and significantly different, respectively.
Results
Characteristics of the studied patients
Seventy-eight COPD patients admitted due to acute exacerbation were randomly recruited for this study. The characteristics of these admitted patients were summarized in Table 1. All subjects had smoking history.
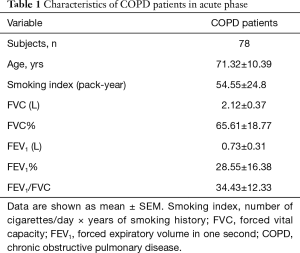
Full table
MUC1 levels in sputum are increased in acute phase of AECOPD
As listed in Table 2, all patients exhibited higher body temperature, respiratory rate, modified British Medical Research Council questionnaire (mMRC) grade and PaCO2, greater blood leukocyte and neutrophil counts, lower blood pH value and PaO2 in acute phase than in remission phase of COPD. The levels of both CT and EC fragments of MUC1 in induced sputum assessed by ELISA were significantly increased in acute phase when compared to remission phase (Figure 1).
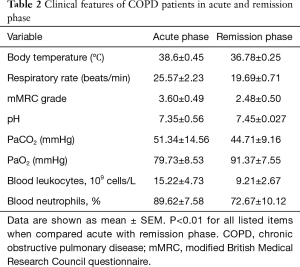
Full table
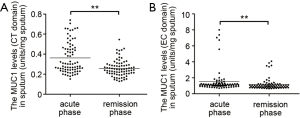
The increases of sputum MUC1 during acute phase of COPD correlate with sputum neutrophil counts, lung function and age
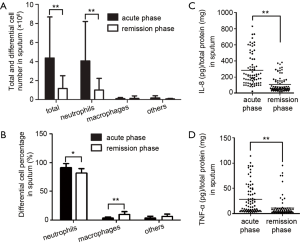
Total and differential cell counting were carried out in the induced sputum samples from patients. Total leukocytes, neutrophils and neutrophil percentage in sputum were significantly higher in acute phase than in remission phase, whereas higher macrophage count and percentage were found in sputum from remission phase (Figure 2A,B), which meant innate immunity occurred in acute phase. Furthermore, significantly higher level of pro-inflammatory cytokines IL-8 and TNF-α were also found in sputum from acute phase than those from remission phase (Figure 2C,D).
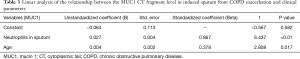
The correlation between MUC1 levels (CT and EC domain) in induced sputum and clinical parameters (age, sputum neutrophil counts, sputum levels of TNF-α and IL-8, and FEV1/FVC) during the acute phase of COPD exacerbation was evaluated by linear regression analysis. The results showed that neutrophil counts in sputum and patients’ age had independently significant effects on the level of sputum MUC1 CT domain (Table 3). FEV1/FVC value and patients’ age had independently significant effects on the level of sputum MUC1 EC domain (Table 4).
Full table

Full table
Muc1 levels are increased in lung tissues and BAL fluids from mice with acute airway inflammation
To verify whether the level of sputum MUC1 is influenced by inflammation during the acute phase of AECOPD, we examined Muc1 expression in lung tissues and BAL fluids from mice developing acute airway inflammation. Mice were given intranasal LPS inhalation and sacrificed at 12, 24, 48 or 96 h or 1 week after. Lung histological analysis showed that lung inflammation was found in alveolar interstitial of LPS-treated mice at 12 and 24 h, relieved at 48 h and clarified completely at 1 week (Figure 3A). In line with the lung inflammation, more leukocytes were found in BAL fluids from LPS-treated mice. After LPS inhalation, the cell counts in BAL fluids reached a peak at 24 h, were maintained on a sub-high level until 96 h and then was decreased but still detectable at 1 w. Most of the infiltrated cells in BAL fluid were neutrophils (Figure 3B).
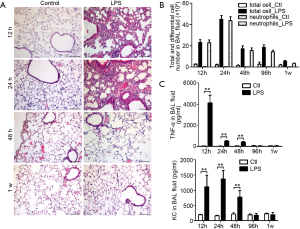
Increased keratinocyte-derived chemokine (KC, mouse ortholog of human IL-8) and TNF-α levels were detected in BAL fluid from mice upon LPS inhalation. The maximum level of TNF-α was observed at 12 h. Then the TNF-α level was kept at a lower level at 24 and 48 h and recovered to basal level at 96 h, a time point at which we could still observe leukocytes infiltration in BAL fluid. Similarly, KC levels in BAL fluids were upregulated at 12, 24 and 48 h and returned to basal level at 96 h and 1 week (Figure 3C).
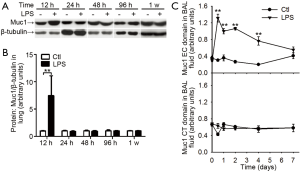
Next, we measured Muc1 levels in lung tissues and BAL fluids with antibodies against CT and EC domain of Muc1, respectively. In whole lung lysates, the levels of Muc1 CT domain were increased at 12 h after LPS inhalation and returned to basal level at 24 h (Figure 4A,B). In BAL fluids, the levels of Muc1 CT fragment were not changed after LPS inhalation; whereas the EC fragment in BAL fluid from LPS-treated mice was rapidly increased at 12 h, and then declined gradually to the basal level in one week (Figure 4C), suggesting differential enhancements of Muc1 fragments in mouse lung during inflammation.
Discussion
The present study demonstrated that both sputum MUC1 CT and EC fragments were increased in acute phase of AECOPD. Linear regression analysis showed that the increment of sputum MUC1 CT fragment level correlated positively with both sputum neutrophil counts and age, and sputum MUC1 EC level correlated positively with obstruction and age. Unlike the situation in AECOPD, only Muc1 EC but not CT fragment was increased in BAL fluid from mice developing acute lung inflammation induced by LPS inhalation. These results suggested that sputum MUC1 changes indicated by EC or CT fragments in AECOPD reflect differential symptoms in acute exacerbation of COPD. The sputum CT fragment level increase can happen under acute lung inflammation with background chronic lung inflammation and indicate the severity of airway inflammatory response, especially at early stage of acute exacerbation. And the sputum EC fragment level increase reflects the lung function decline in AECOPD.
COPD is characterized by progressive lung function impairment and frequent exacerbation correlated with severity of this disease which serves the major reason for hospital admission (2). One subtype of MUC1 protein KL-6 were reportedly increased in the lungs, plasma and sputum from stable COPD patients and affected by age and cigarette smoking (12). MUC1 is a membrane-tethered mucin expressed on the surface of epithelial cells lining mucosal surfaces. The full-length MUC1 contains a very large EC domain (18) where the tandem repeat provides a scaffold on which oligosaccharide structures stand, a hydrophobic membrane-spanning domain (SEA domain), and a phosphorylated CT domain containing signaling motifs. Autocleavage within SEA domain (58 amino acids upstream of the TE domain) occurs immediately after translation and generates two fragments maintaining association via non-covalent linkage (4). In this study, though by using a direst ELISA assay, which is less sensitive and specific than sandwich ELISA assay, both MUC1 CT and EC fragments were detectable in sputum from COPD patients and correlated positively with patients’ age, being consistent with previous report (12). The positively correlation between CT fragment increase and neutrophil counts means that CT fragment increase in sputum is affected by airway inflammation in AECOPD which was judged by the significantly higher level of pro-inflammatory cytokines IL-8 and TNF-α in sputum from acute phase than those from remission phase (Figure 2C,D).
Muc1 overexpression in airway epithelial cells under pulmonary bacterial infection was considered as an immune response which in turn inhibits excessive inflammation and could be controlled by proinflammatory cytokine like TNF-α (9,19,20). AECOPD is mainly caused by bacterial infection in the airway and lung. However, CT fragment increase was not found in BAL fluid, though CT fragment increase was identified in lungs from mice undergoing LPS induced acute lung inflammation. Since MUC1 CT fragment is tethered on the cell membrane, most of MUC1 CT fragment in sputum or in BAL fluid could come from detached epithelial cells induced by airway damage. Therefore, we propose that LPS inhalation in this study caused lung inflammation but did not induced enough airway damage to cause release of Muc1 CT fragment carrying epithelial cells into BAL fluid.
The increase of sputum MUC1 CT fragment in AECOPD was probably due to detachment of damaged epithelial cells carrying overexpressed MUC1 caused by background chronic inflammation plus a flare up of inflammation in acute exacerbation, since hallmark features of COPD include obstructive bronchiolitis and emphysema parenchymal destruction (21,22). In order to maintain the epithelial integrity, the detachment of damaged cells from the monolayer results from extrusion effects by de-differentiated ciliated cells (23). We noticed that CT fragment was rapidly increased and recovered at 12 h after LPS inhalation, a time frame before other inflammatory phenotypes disappeared and could be taken as early stage of lung inflammation. Thus, in case of COPD exacerbation, the sputum MUC1 CT level indicated the intensity of host inflammatory response during acute phase and the airway damage/repair in AECOPD.
Unlike CT fragment, EC fragment was increased in sputum from AECOPD and in BAL fluid from mice with LPS induced lung inflammation. In LPS-inhaled mice, EC fragments in mice BAL fluid were rapidly increased at 12 h post LPS inhalation and followed by a gradual decline lasting for 7 days (Figure 4C), which mean that the release of EC fragment into BAL fluid probably depended on the inflammatory environment. Muc1 EC domain is the component of mucus and was supposed to be the adhesion site of bacteria (4,24,25). The mucus with bacterial burden will be cleared from the mucosal surface by cilia beating (26). Under inflammatory environment, the sputum EC fragments came from a neutrophil elastase mediated degradation of EC MUC1 on the surface of airway epithelial cells (11). However, excessive mucus production will contribute to airway blockage and lung functions decline, a situation consistent with positive correlation between sputum MUC1 EC fragment level and obstruction (determined by FEV1/FVC value reduction) revealed by linear regression analysis (Table 4). Therefore, we proposed sputum EC fragment level change reflect a potential change on lung function status in COPD exacerbation.
In summary, we showed that sputum MUC1 was increased in the acute phase of AECOPD. The sputum MUC1 increase is likely due to protein overexpression and release incurred by inflammation. The change of various sputum MUC1 fragments may serve as indicators for evaluating inflammatory response and the lung function recover of COPD exacerbation. However, we could not ignore the limitation in methods we took in this study. The redox reagent dithiothreitol used in sputum lysis might impact the ELISA, though we diluted the sputum lysate in ELISA to avoid such possible interferences. Due to the lack of commercial antibody pairs and commercial standards for sandwich ELISA of MUC1 EC and CT domain, the results obtained in this study only illustrated the change tendency of MUC1 in development and relief of AECOPD. As a protein having multi-functions, it is highly possible that MUC1 exerts more complex functions in COPD. The association between MUC1 changes and AECOPD, and the clinical implications of sputum MUC1 measurement need more studies to validate in future.
Acknowledgements
We thank Professor Kefang Lai (Guangzhou Medical University, Guangzhou, China) for providing training in collection and analysis of induced sputum.
Funding: This work was supported by National Natural Science Foundation of China (81220108001), the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2014) and Guangzhou Department of Education Team Grant for Innovation (13C08).
Footnote
Conflicts of Interest: Clinical trial—this study is registered at Chinese Clinical Trial Registry (http://www.chictr.org) with registration number ChiCTR-CCS-13003116.
Ethical Statement: The study was approved by the ethic committee of The First Affiliated Hospital of Guangzhou Medical University and written informed consent was obtained from all patients.
References
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2011. Available online: www.gold.com
- Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J 2007;29:1224-38. [Crossref] [PubMed]
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008;359:2355-65. [Crossref] [PubMed]
- Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia 2001;6:339-53. [Crossref] [PubMed]
- Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem 1990;265:15286-93. [PubMed]
- Lan MS, Batra SK, Qi WN, et al. Cloning and sequencing of a human pancreatic tumor mucin cDNA. J Biol Chem 1990;265:15294-9. [PubMed]
- Ligtenberg MJ, Vos HL, Gennissen AM, et al. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J Biol Chem 1990;265:5573-8. [PubMed]
- Lu W, Hisatsune A, Koga T, et al. Cutting edge: Enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol 2006;176:3890-4. [Crossref] [PubMed]
- Li Y, Dinwiddie DL, Harrod KS, et al. Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 2010;298:L558-63. [Crossref] [PubMed]
- Choi S, Park YS, Koga T, et al. TNF-alpha is a key regulator of MUC1, an anti-inflammatory molecule, during airway Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol 2011;44:255-60. [Crossref] [PubMed]
- Kuwahara I, Lillehoj EP, Hisatsune A, et al. Neutrophil elastase stimulates MUC1 gene expression through increased Sp1 binding to the MUC1 promoter. Am J Physiol Lung Cell Mol Physiol 2005;289:L355-62. [Crossref] [PubMed]
- Ishikawa N, Mazur W, Toljamo T, et al. Ageing and long-term smoking affects KL-6 levels in the lung, induced sputum and plasma. BMC Pulm Med 2011;11:22. [Crossref] [PubMed]
- Ko FW, Ip M, Chan PK, et al. A 1-year prospective study of the infectious etiology in patients hospitalized with acute exacerbations of COPD. Chest 2007;131:44-52. [Crossref] [PubMed]
- Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418-22. [Crossref] [PubMed]
- Patel IS, Seemungal TA, Wilks M, et al. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002;57:759-64. [Crossref] [PubMed]
- Collaborating Research Group for Noninvasive Mechanical Ventilation of Chinese Respiratory Society. Pulmonary infection control window in treatment of severe respiratory failure of chronic obstructive pulmonary diseases: a prospective, randomized controlled, multi-centred study. Chin Med J (Engl) 2005;118:1589-94. [PubMed]
- Pizzichini E, Pizzichini MM, Efthimiadis A, et al. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med 1996;154:308-17. [Crossref] [PubMed]
- Gendler SJ, Burchell JM, Duhig T, et al. Cloning of partial cDNA encoding differentiation and tumor-associated mucin glycoproteins expressed by human mammary epithelium. Proc Natl Acad Sci U S A 1987;84:6060-4. [Crossref] [PubMed]
- McAuley JL, Linden SK, Png CW, et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest 2007;117:2313-24. [Crossref] [PubMed]
- Koga T, Kuwahara I, Lillehoj EP, et al. TNF-alpha induces MUC1 gene transcription in lung epithelial cells: its signaling pathway and biological implication. Am J Physiol Lung Cell Mol Physiol 2007;293:L693-L701. [Crossref] [PubMed]
- Kang MJ, Choi JM, Kim BH, et al. IL-18 induces emphysema and airway and vascular remodeling via IFN-gamma, IL-17A, and IL-13. Am J Respir Crit Care Med 2012;185:1205-17. [Crossref] [PubMed]
- Thurlbeck WM. Pathology of chronic airflow obstruction. Chest 1990;97:6S-10S. [Crossref] [PubMed]
- Henson PM, Vandivier RW, Douglas IS. Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proc Am Thorac Soc 2006;3:713-7. [Crossref] [PubMed]
- Lillehoj EP, Hyun SW, Kim BT, et al. Muc1 mucins on the cell surface are adhesion sites for Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2001;280:L181-7. [PubMed]
- Lillehoj EP, Kim BT, Kim KC. Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. Am J Physiol Lung Cell Mol Physiol 2002;282:L751-6. [Crossref] [PubMed]
- Kesimer M, Ehre C, Burns KA, et al. Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol 2013;6:379-92. [Crossref] [PubMed]


