Cardiovascular benefits of the newer medications for treating type 2 diabetes mellitus
Introduction
Diabetes mellitus is growing in pandemic proportions and is associated with significant morbidity, mortality, and health care expenditures. It affects more than 29 million people in the United States (9.3% population), a trend if continues can result in every third adult having diabetes mellitus by 2050 (1). It affects around 415 million people worldwide, a number that is projected to increase to 642 million worldwide (12% population) by 2040, affecting one in every 10 adults (2). In the Unites States diabetes mellitus is the seventh leading cause of death, a number that is underreported (1). Per the International Diabetes Federation, 1 person dies from diabetes mellitus every 6 seconds around the world (2). The total cost of diabetes mellitus is estimated to be more than $245 billion in the United States and around $673 billion worldwide (1,2). Type 2 diabetes mellitus (T2DM) is the most common, accounting for about 90–95% of diagnosed diabetes in United States adults (1).
Individuals with T2DM have a 2- to 3-fold increased risk of cardiovascular (CV) events compared with their non-diabetic counterparts, and CV mortality is responsible for around 80% of the mortality in T2DM (3,4). A meta-analysis showed that the relative risk (RR) for coronary heart disease or stroke has been estimated at 1.18% for every 1% increase in glycated hemoglobin (HbA1C) (5). Available evidence suggests that hyperglycemia is a weak risk factor for CV events, and tight glycemic control had very little effect on reducing macrovascular events and CV-related death (6,7). Intensive glycemic control increased mortality without reducing CV events (8). In addition to hyperglycemia, individuals with T2DM can have other features of insulin resistance-metabolic syndrome like hypertension, lipid abnormalities, and obesity which are all associated with increased CV disease and stroke risk even in the absence of T2DM (9,10). Thus, the management of T2DM patients should include control of CV risk factors in addition to hyperglycemia to improve both macrovascular and microvascular complications and resulting morbidity and mortality. With the increasing prevalence of T2DM and metabolic syndrome, and associated significant CV disease and mortality, there is a need for novel diabetic medications which have a role in improving metabolic risk factors in addition to hyperglycemia.
Metformin and pioglitazone has been shown to have a CV protective benefit via their effect on improving lipid profile, weight, and blood pressure (BP), and insulin sensitivity (11,12). Metformin decreases gluconeogenesis in liver, increases insulin sensitivity, and improves the efficacy of endogenous insulin (11). A meta-analysis suggesting that rosiglitazone was associated with significantly increased risk of myocardial infarction (MI) and CV-related death led to an intense debate about the CV safety of the antidiabetic drugs (13). This led to regulatory agencies requiring CV safety trials as part of the approval process for newer antidiabetic drugs (14,15). This review article will discuss the CV benefits of the newer incretin based therapies and sodium glucose cotransporter-2 (SGLT-2) inhibitors as observed in their CV safety trials.
Incretin based therapies
Incretins are insulinotropic intestinal hormones secreted into the blood by the enteroendocrine cells in response in oral glucose intake (16). These hormones then stimulate the secretion of insulin from the pancreatic ß-cells, a phenomenon called the incretin effect (16). Two incretins, glucose-dependent insulinotropic peptide and glucagon-like peptide 1 (GLP-1) were characterized and studied in human beings. Both the incretin hormones are rapidly degraded by dipeptidyl peptidase-4 (DPP-4) in the circulation (16). The insulinotropic effect of glucose-dependent insulinotropic peptide is deficient in patients with T2DM thereby limiting it from being a therapeutic target (16). The incretin effect of GLP-1 is better preserved in T2DM patients, thus making it a potential therapeutic target in this population (16). GLP-1 also inhibits gastric emptying, decreases appetite, inhibits glucagon secretion from pancreatic α-cells via a paracrine effect medicated by somatostatin release, and slows the rate of endogenous glucose production, all of which improve glycemic control in T2DM (16). Two approaches were devised to augment the beneficial role on incretins in T2DM. One approach is the use of exogenous GLP-1 agonists resistant to degradation by DPP-4. The other approach is the use of DPP-4 inhibitors to enhance the half-life and effects of endogenous GLP-1.
GLP-1 receptor agonists (RAs)
Currently available RAs include albiglutide once weekly, dulaglutide once weekly, exenatide twice daily, extended release exenatide once weekly, liraglutide once daily, and lixisenatide once daily. A comprehensive review of all head-to-head data indicates that liraglutide followed by exenatide appear to offer the best HbA1C (approximately 1–2%) and weight reduction (17). Retrospective analyses of data suggested a possible reduced likelihood of having a CV event over a 1- to 4-year period among patients who were treated with exenatide twice daily compared with other glucose-lowering agents (18). In 2 recent large randomized controlled trials, the GLP-1 RAs liraglutide and semaglutide showed remarkable CV benefit compared to placebo when added to patients with T2DM and high CV risk (19,20). The perceived CV benefit of GLP-1 RAs can be attributed to the extra-pancreatic pleotropic effects of GLP-1 on the CV system, and the favorable impact of GLP-1 RAs on important non-glycemic CV risk factors like BP, weight, and lipid profile.
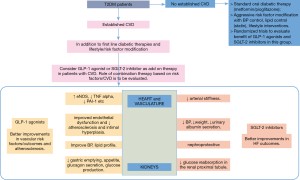
GLP-1 receptors are present on rodent and human cardiac myocytes, endothelial cells, and vascular smooth muscle cells (16). Numerous in vitro studies have demonstrated the protective effects of liraglutide and exenatide on endothelial cells independent of GLP-1 RA glycemic and weight reduction effects (21,22). The favorable effects on endothelial cells is mediated via activation of endothelial nitric oxide synthase, and inhibition of tumor necrosis factor alpha, plasminogen activator inhibitor-1, various growth factors, and adhesion molecules. Through these GLP-1 receptor-dependent mechanisms, GLP-1 RAs improve endothelial dysfunction and attenuate atherosclerosis and intimal hyperplasia. Exenatide and liraglutide lowered BP, reduced myocardial infarct size, and improved systolic and diastolic cardiac function in various animal models of ischemia-reperfusion injury and congestive heart failure (HF) (16,21,22). Infusion of exenatide during two consecutive days in men with T2DM and HF led to significantly increased cardiac index and decreased pulmonary capillary wedge pressure compared to placebo (23) (Figure 1).
The CV benefit of GLP-1 RAs in terms of risk factor improvement compared with placebo and most standard anti-diabetic agents was observed in human clinical studies. Treatment with liraglutide or exenatide was associated with modest systolic BP reductions (21). In a meta-analysis of 33 trials (12,469 patients) in which 41% patients were treated with liraglutide and the rest with exenatide, GLP-1 RA treatment achieved a greater systolic BP reduction than comparator therapy [weighted mean difference of 2.22 mmHg, 95% confidence interval (CI): −2.97 to −1.47] independent of baseline BP, weight loss, or improvement in HbA1C (24). Improvements in lipid profile by reducing triglycerides, apolipoproteins B-48, free fatty acids, low-density lipoprotein cholesterol (LDL-C), and total cholesterol was observed with GLP-1 RA treatment (21). In a meta-analysis of 35 trials, GLP-1 RAs were associated with modest reductions in LDL-C, total cholesterol, and triglycerides but no significant improvement in high density lipoprotein cholesterol (HDL-C) (25). Clinical studies of liraglutide and exenatide also demonstrated significant weight loss, and improvements in CV risk biomarkers like plasminogen activator inhibitor-1 and high-sensitivity C-reactive protein (16,21).
The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial randomized 606 T2DM patients who had a MI or who had been hospitalized for unstable angina within the previous 180 days to receive lixisenatide or placebo in addition to locally determined standards of care (26). Primary end-point was a composite of CV death, MI, stroke, or hospitalization for unstable angina. At 25-month follow up, the primary end-point event occurred in 13.4% patients in the lixisenatide group and in 13.2% in the placebo group [hazard ratio (HR) 1.02; 95% CI: 0.89 to 1.17], which showed the noninferiority of lixisenatide to placebo (P<0.001) but did not show superiority (P=0.81). There were no significant between-group differences in the rate of hospitalization for HF (HR in the lixisenatide group, 0.96; 95% CI: 0.75 to 1.23) or the rate of death (HR, 0.94; 95% CI: 0.78 to 1.13). The authors concluded that the addition of lixisenatide to usual care did not significantly alter the rate of major CV events or other serious adverse events (26).
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial is a double-blind, placebo controlled trial that randomized 9340 T2DM patients at high CV risk to either 1.8 mg (or the maximum tolerated dose) of liraglutide or matching placebo once daily as a subcutaneous injection in addition to standard care (19). High CV risk was defined as age ≥50 years with at least one CV coexisting condition (coronary heart disease, cerebrovascular disease, peripheral vascular disease, chronic kidney disease of stage 3 or greater, or chronic HF of New York Heart Association class II or III) or an age ≥60 years with at least one CV risk factor (microalbuminuria or proteinuria, hypertension and left ventricular hypertrophy, left ventricular systolic or diastolic dysfunction, or an ankle-brachial index of less than 0.9). The primary composite outcome in the time-to-event analysis was the first occurrence of death from CV causes, nonfatal MI, or nonfatal stroke. After a median follow up of 3.8 years, significantly fewer patients in the liraglutide group than in the placebo group experienced the primary outcome (13.0% vs. 14.9%; HR 0.87; 95% CI: 0.78 to 0.97; P<0.001 for noninferiority; P=0.01 for superiority), CV-related death (4.7% vs. 6.0%; HR 0.78; 95% CI: 0.66 to 0.93; P=0.007), or all-cause death (8.2% vs. 9.6%; HR 0.85; 95% CI: 0.74 to 0.97; P=0.02). In subgroup analysis, the CV benefit of liraglutide was more apparent in patients with established CV disease compared to placebo (14% vs. 16.7%; HR 0.83; 95% CI: 0.74 to 0.93; P=0.04).
The liraglutide group had non-significantly lower frequencies of nonfatal MI, nonfatal stroke, HF hospitalization rate, and acute pancreatitis. At 36 months, the liraglutide group had improved CV risk factor profile in terms of higher weight loss (mean 2.3 kg; 95% CI: 2.5 to 2.0), lower systolic BP (1.2 mmHg; 95% CI: 1.9 to 0.5), and a lower rate of nephropathy events (1.5 vs. 1.9 events per 100 patient-years of observation; HR 0.78; 95% CI: 0.67 to 0.92; P=0.003). The heart rate was 3.0 beats per minute (95% CI: 2.5 to 3.4) higher in the liraglutide group (19). The composite outcome of renal or retinal microvascular events was lower in the liraglutide group than in the placebo group (HR, 0.84; 95% CI: 0.73 to 0.97; P=0.02), a difference that was driven by a lower rate of nephropathy events in the liraglutide group (1.5 vs. 1.9 events per 100 patient-years of observation; HR, 0.78; 95% CI: 0.67 to 0.92; P=0.003). Retinopathy events were numerically higher in the liraglutide group (0.6 vs. 0.5 events per 100 patient-years; HR, 1.15; 95% CI: 0.87 to 1.52; P=0.33) (19). Hypoglycemic events and gastrointestinal side effects (nausea, vomiting, diarrhea, abdominal discomfort, cholelithiasis, and acute cholecystitis) were significantly more frequent in the liraglutide group (19).
Semaglutide, a GLP-1 analogue with an extended half-life of approximately 1 week is currently in development but not yet approved for the T2DM. The Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) randomized 3,297 T2DM patients with either CV disease (89.3%) or chronic kidney disease (10.7%) to once-weekly subcutaneous semaglutide (0.5 or 1.0 mg) or placebo for 104 weeks in addition to standard-care regimen (20). The primary composite outcome was the first occurrence of CV death, nonfatal MI, or nonfatal stroke. After a median follow up of 2.1 years, the primary outcome (6.6% in semaglutide group vs. 8.9% in placebo group; HR, 0.74; 95% CI: 0.58 to 0.95; P<0.001 for noninferiority) occurred in significantly fewer semaglutide patients than placebo patients. There were significantly fewer nonfatal stroke events (1.6% vs. 2.7%; HR, 0.61; 95% CI: 0.38 to 0.99; P=0.04) and numerically fewer nonfatal MI events (2.9% vs. 3.9%; HR 0.74; 95% CI: 0.51 to 1.08; P=0.12) in the semaglutide group. Rates of CV-related death were similar in the two groups. The mean HbA1C in the semaglutide group, as compared with the placebo group, was 0.7 percentage points lower in the group receiving 0.5 mg and 1.0 percentage point lower in the group receiving 1.0 mg (estimated treatment difference) (P<0.001 for both comparisons). At 104 weeks, the semaglutide group had improved CV risk factor profile in terms of higher mean weight loss (2.9 kg lower in the group receiving 0.5 mg and 4.3 kg lower in the group receiving 1.0 mg; P<0.001 for both comparisons), lower mean systolic BP (1.3 mmHg lower in the group receiving 0.5 mg, P=0.10; and 2.6 mmHg lower in the group receiving 1.0 mg, P<0.001).
The semaglutide group had a lower rate of nephropathy events (3.8% vs. 6.1%; HR 0.64; 95% CI: 0.46 to 0.88; P=0.005). The mean heart rate was 2.0 bpm higher in the group receiving 0.5 mg semaglutide and 2.5 bpm higher in the group receiving 1.0 mg semaglutide (P<0.001 for both comparisons) compared to placebo (20). Diabetic retinopathy complications occurred in more semaglutide treated patients (3% vs 1.8%; HR 1.76; 95% CI: 1.11 to 2.78; P=0.02), and were seen very early in the trial (20). Although the overall number of retinopathy events was low, there was an unexpected higher rate of retinopathy complications (vitreous hemorrhage, blindness, or the need for treatment with an intravitreal agent or photocoagulation) in the semaglutide group. It must be reminded that SUSTAIN-6 is a preapproval trial with a relatively short duration (2.1 years) designed to test the non-inferiority of semaglutide compared to placebo in influencing CV events, but not to investigate the superiority of semaglutide in reducing CV events. Results from this trial are encouraging and hypothesis generating for future research.
DPP-4 inhibitors
DPP-4 inhibitors are oral diabetic medications that prevent the peripheral inactivation of incretins by DPP-4, resulting in increased half-life, and extended insulinotropic and other actions of GLP-1 in T2DM patients (27). Currently approved DPP-4 inhibitors are sitagliptin, saxagliptin, linagliptin, alogliptin, and vildagliptin. DPP-4 has many substrates and thus inhibition of DPP-4 may have diverse effects in addition to prolongation of incretin effect. DPP-4 can also degrade inflammatory chemokines, neuropeptides, and vasodilatory/fibrinolytic peptides like substance P and bradykinin (27). Although available data from early animal and human studies showed a favorable effect of DPP-4 inhibition on atherosclerosis, the effects on endothelial function, vasodilatation, cardiac remodeling, and cardiac function are inconsistent (27,28). DPP-4 inhibition was associated with improved glycemic control, improved total cholesterol and triglyceride levels, and weight neutrality (27,29). The effects of DPP-4 inhibition on CV disease risk factors, cardiac function, and vascular repair likely represent contributions from and a balance of both GLP-1-dependent actions of DPP-4 inhibitors as well as mechanisms independent of GLP-1 (27). Recent large clinical trials have confirmed the safety and neutral effect of DPP-4 inhibitors on CV outcomes.
The Saxagliptin Assessment of Vascular Outcomes recorded in patients with diabetes mellitus-Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) study randomized 16,492 patients with T2DM with established CV disease or multiple risk factors for vascular disease, to receive either daily saxagliptin or placebo in addition to standard diabetic and CV disease therapy (30). At median follow up of 2.1 years, saxagliptin did not increase or decrease the risk of the primary composite end-point of CV death, non-fatal MI or non-fatal ischemic stroke, compared with placebo (HR, 1.00; 95% CI: 0.89 to 1.12; P<0.001 for non-inferiority and P=0.99 for superiority). The occurrence of the major secondary end-point of a composite of CV death, MI, stroke, hospitalization for unstable angina, coronary revascularization, or HF was similar between the two groups (12.8% in saxagliptin group vs. 12.4% in placebo group; HR, 1.02; 95% CI: 0.94 to 1.11; P=0.66). More patients in the saxagliptin group than in the placebo group were hospitalized for HF (3.5% vs. 2.8%; HR, 1.27; 95% CI: 1.07 to 1.51; P=0.007) (30). A meta-analysis of randomized trials of DPP-4 inhibitors showed that saxagliptin was significantly associated with a 21% increased risk of HF (RR, 1.215; 95% CI: 1.028 to 1.437; P=0.022) (31). The EXAmination of CV outcoMes with alogliptIN versus standard of carE (EXAMINE) trial randomized 5,380 T2DM patients with either an acute MI or unstable angina requiring hospitalization within the previous 15 to 90 days to receive alogliptin or placebo in addition to existing antihyperglycemic and CV drug therapy for a median follow up of 40 months (32). At 18-month follow up, alogliptin did not increase or decrease the risk of the primary composite end-point of CV death, non-fatal MI or non-fatal stroke, compared with placebo (11.3% vs. 11.8%; HR, 0.96; upper boundary of the one-sided repeated CI, 1.16; P<0.001 for non-inferiority; P = 0.32 for superiority). The analysis of the principal secondary end-point of death from CV causes, nonfatal MI, nonfatal stroke, or urgent revascularization due to unstable angina showed no significant difference between the alogliptin group and the placebo group (12.7% vs. 13.4%; HR, 0.95; upper boundary of the one-sided repeated CI, 1.14) (32). In a post hoc analysis, alogliptin did not significantly increase the rates of HF hospitalization compared to placebo although a numerical increase was noted (3.9% vs. 3.3%; HR, 1.19; 95% CI: 0.90 to 1.58) (33). Alogliptin had no effect on composite events of CV and hospital admission for HF in a post hoc analysis (HR 1·00; 95% CI: 0.82 to 1.21) and results did not differ by baseline b-type natriuretic peptide concentration (34). An increase in hospitalization for HF with alogliptin was noted in patients without a prior history of HF (2.2% with alogliptin vs. 1.3% with placebo; HR, 1.76; P=0.026) (34). Rates of CV death were similar between alogliptin and placebo (4.1% vs. 4.9%, HR 0.85; 95% CI: 0.66 to 1.10) (35).
The Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) evaluated the long-term CV safety of the DPP-4 sitagliptin in T2DM patient with CV disease (36). TECOS randomized 14,671 T2DM patients with established CV disease (history of major coronary artery disease, ischemic cerebrovascular disease, or atherosclerotic peripheral arterial disease) to either daily oral sitagliptin or matching placebo in addition to existing therapy (36). At median follow up of 3 years, sitagliptin was noninferior to placebo for the primary composite end-point of CV death, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina (11.4% vs. 11.6%; HR, 0.98; 95% CI: 0.88 to 1.09; P<0.001; HR in the intention-to-treat analysis, 0.98; 95% CI: 0.89 to 1.08; P=0.65 for superiority). Similarly, sitagliptin was noninferior to placebo for the secondary composite end-point of first confirmed event of CV death, nonfatal MI, or nonfatal stroke (HR in the per-protocol analysis, 0.99; 95% CI: 0.89 to 1.11; P<0.001 for noninferiority; HR in the intention-to-treat analysis, 0.99; 95% CI: 0.89 to 1.10; P=0.84 for superiority). Sitagliptin did not increase the rates of HF hospitalization (1.07 per 100 person-years vs. 1.09 per 100 person-years; HR, 1.00; 95% CI: 0.83 to 1.20; P=0.98) (36). The TECOS confirmed that adding sitagliptin to usual care did not appear to increase the risk of major adverse CV events, especially hospitalization for HF, or other adverse events.
In a pooled analysis of randomized trials in T2DM patients comparing linagliptin to either placebo or an active comparator medication, linagliptin was not associated with an increased CV risk (37). The 27% increased risk of HF hospitalization with the DPP-4 inhibitor saxagliptin as observed in the SAVOR-TIMI 53 trial was not replicated in the EXAMINE or the TECOS trials with alogliptin and sitagliptin, respectively (38). The Canadian Network for Observational Drug Effect Studies (CNODES) investigators examined existing data from multiple cohorts of patients (total of 1,499,650 patients, with 29,741 hospitalized for HF) to determine whether the use of incretin-based drugs, as compared with oral antidiabetic-drug combinations, in routine clinical practice is associated with an increased risk of HF (38). In this retrospective observational analysis, incretin-based therapy did not increase the rate of hospitalization for HF among patients with a history of HF (HR, 0.86; 95% CI: 0.62 to 1.19) or among those without a history of HF (HR, 0.82; 95% CI: 0.67 to 1.00). These results were similar for both DPP-4 inhibitors and GLP-1 RAs. The investigators concluded that incretin-based drugs were not associated with an increased risk of hospitalization for HF, as compared with commonly used combinations of oral antidiabetic drugs (38).
In a meta-analysis of 54 randomized controlled trials of DPP-4 inhibitors in 74,737 participants with a minimum follow-up of 12 weeks, DPP-4 inhibitors were not associated with an increased risk of HF compared to comparators (RR 1.106; 95% CI: 0.995 to 1.228; P=0.062) (31). The authors noted a differential effect of each DPP-4 inhibitor on the risk of HF. Use of saxagliptin significantly increased the risk of HF by 21% especially among patients with high CV risk (RR 1.215; 95% CI: 1.028 to 1.437; P=0.022), while others were not associated with an increased HF risk (31). Age ≥65 years, diabetes duration of ≥10 years and BMI ≥30 kg/m2 were associated with an increased risk of HF among patients using saxagliptin (31). Based on available data, the risk of HF with DPP-4 inhibitors is of definitive concern and appears to be drug-specific. After reviewing the data from SAVOR-TIMI 53 and EXAMINE trials, the FDA found an increased risk of HF with saxagliptin and alogliptin particularly in patients with underlying heart or kidney disease and announced safety warnings to be added to the labels of these two DPP-4 inhibitors (39).
SGLT2 inhibitors
SGLT-2 inhibitors are a novel class of medications that improve glycemic control via reduced glucose reabsorption in the renal proximal tubule resulting in glycosuria (40). SGLT-2 is present in segment 1 of the proximal tubule and normally accounts for around 90% of the glucose reuptake (40). As SGLT-2 expression is highly specific for the kidneys, SGLT-2 inhibitors should not affect glucose transport in other tissues (41). SGLT-2 inhibitors act independent of insulin secretion, severity of insulin resistance, and pancreatic ß-cell failure (40). The above unique mechanisms are responsible for the very low potential of SGLT-2 inhibitors to cause hypoglycemia and suggest that they may be effective across the spectrum of T2DM disease progression. As they act within the tubule, reduced glomerular filtration rate in chronic kidney disease can reduce the efficacy and safety of SGLT-2 inhibitors (40,41). Canagliflozin, dapagliflozin, and empagliflozin are some of the approved SGLT-2 inhibitors.
SGLT-2 inhibitors improve both glycemic and non-glycemic risk factors in T2DM patients. They induce urinary glucose losses around 40–80 g/day, resulting in decent glycemic control (HbA1c reduction around 0.7%) (42). This corresponds to around 200–300 kilocalories daily which can result in a 2–3 kg body weight loss over 24–52 weeks (40). Most weight loss associated with SGLT-2 inhibition was due to reduction in visceral fat which is associated with increased risk of T2DM, CV complications and overall mortality (40). SGLT-2 inhibitors showed a consistent BP lowering effect, more systolic than diastolic, without a compensatory increase in pulse rate, in multiple clinical studies (43,44). The mechanism of BP reduction is not entirely clear. Possible mechanisms include the osmotic diuretic effect, weight reduction, and a possible direct vascular effect via reducing arterial stiffness (40,44). In a meta-analysis of 27 randomized controlled trials (n=12,960 participants), SGLT-2 inhibitors significantly reduced both systolic BP (weighted mean difference of 4.0 mmHg) and diastolic BP (weighted mean difference of 1.6 mmHg) from baseline (43). SGLT-2 inhibitors were associated with a small increase in both HDL-C and LDL-C levels with concomitant reductions in triglyceride levels, the effect of which on CV events is not very clear at this time (40,45). SGLT-2 inhibition is also associated with reduced glomerular hyperfiltration and urinary albumin excretion which might suggest the nephroprotective effect of this class of drugs (40 (Figure 1).
Several analyses of pooled data suggest that SGLT-2 inhibitors do not appear to increase CV risk (40). The best available data on the effects of SGLT-2 inhibition on CV outcomes comes from the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) study (46). The EMPA-REG OUTCOME trial randomized 7020 T2DM patient with established CV disease to receive either 10 or 25 mg of empagliflozin or placebo once daily in addition to standard anti-hyperglycemic and CV disease therapies (46). The primary outcome was a composite of death from CV causes, nonfatal MI (excluding silent MI), or nonfatal stroke. After a median 3.1 years, significantly fewer patients in the empagliflozin group than in the placebo group experienced the primary outcome (10.5% vs. 12.1%; HR in the empagliflozin group, 0.86; 95.02% CI: 0.74 to 0.99; P=0.04 for superiority), which was largely driven by the significantly lower CV-related death (3.7% vs. 5.9%; 38% RR reduction; HR, 0.62; 95% CI: 0.49 to 0.77; P<0.001). There were no significant between-group differences in the occurrence of MI or stroke (P=0.22). The empagliflozin group had lower rates of hospitalization for HF (2.7% vs. 4.1% in placebo group; 35% RR reduction), and death from any cause (5.7% vs. 8.3% in placebo group; 32% RR reduction; HR, 0.68; 95% CI: 0.57 to 0.82, P<0.001). The key secondary outcome which was a composite of the primary outcome plus hospitalization for unstable angina occurred in fewer empagliflozin treated patients (12.8% vs. 14.3% in the placebo group; HR, 0.89; 95% CI: 0.78 to 1.01; P<0.001 for noninferiority and P=0.08 for superiority). Empagliflozin, as compared with placebo, was associated with small reductions in weight, waist circumference, uric acid level, and systolic and diastolic BP with no increase in heart rate and small increases in both LDL-C and HDL-C (46).
Studies examining renal outcomes with SGLT-2 inhibitors were also reported. In a study of 1450 T2DM patients on metformin randomly assigned to either canagliflozin or glimepiride, canagliflozin treated patients had significantly less decline in estimated glomerular filtration rate and greater reduction in urinary albumin-to-creatinine ratio compared to glimepiride (47). The authors concluded that canagliflozin, compared with glimepiride, slowed the progression of renal disease over 2 years in patients with T2DM, and canagliflozin may confer renoprotective effects independently of its glycemic effects (47). This study was not designed nor powered to compare the renoprotective effects of canagliflozin versus glimepiride, so the results should be interpreted as hypothesis-generating (47). Results of a prespecified secondary end-point of the EMPA-REG OUTCOME trial to evaluate the effects of empagliflozin on microvascular outcomes were later published (48). Among patients with glomerular filtration rates of ≥30 mL/minute/1.73 m2, incident or worsening nephropathy occurred in 12.7% patients in the empagliflozin group and in 18.8% patients in the placebo group (HR in the empagliflozin group, 0.61; 95% CI: 0.53 to 0.70; P<0.001), doubling of the serum creatinine level occurred in 1.5% in the empagliflozin group and in 2.6% in the placebo group (significant RR reduction of 44%), renal-replacement therapy was initiated in 0.3% in the empagliflozin group and in 0.6% in the placebo group (55% lower RR). Empagliflozin did not prevent development of microalbuminuria (48). Studies evaluating the CV benefit of canagliflozin [Canagliflozin Cardiovascular Assessment Study (CANVAS)], dapagliflozin [The Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58)], and ertugliflozin in T2DM patients are currently undergoing (49-51).
Update on pioglitazone
Pioglitazone, a thiazolidinedione, acts by regulating peroxisome proliferator-activated receptor-gamma mediated gene expression resulting in increased insulin sensitivity and glucose utilization, and decreased glucose production (12). Pioglitazone has been shown to have a CV protective benefit in diabetic patients via its effect on improving lipid profile, weight, BP, and insulin sensitivity (12). The CV effects of pioglitazone in non-diabetic patients with insulin resistance has been recently investigated., In the recent, double-blind, controlled, Insulin Resistance Intervention after Stroke (IRIS) study which randomized 3,876 non-diabetic patients with insulin resistance and a recent ischemic stroke or transient ischemic attack to receive either pioglitazone or placebo, pioglitazone reduced the primary outcome of fatal or nonfatal stroke or MI (9% vs. 11.8% in the placebo group; HR in the pioglitazone group, 0.76; 95% CI: 0.62 to 0.93; P=0.007), without reducing all-cause mortality at 4.8-year follow-up (52). Pioglitazone also reduced incident diabetes mellitus (3.8% vs. 7.7% in the placebo group; HR, 0.48; 95% CI: 0.33 to 0.69; P<0.001) (52). These findings further support the fact that improving metabolic control irrespective of the presence on diabetes mellitus has an important role to play in improving atherosclerotic risk factors and resultant CV disease in high risk patients.
Role of incretin based therapies and SGLT-2 inhibition in the future of T2DM and CV disease
Recent years have been exciting for both cardiologists and endocrinologists with the introduction of newer therapies for T2DM with observed CV benefits in large randomized trials. The primary outcome in these trials was a CV composite of CV-related death, non-fatal MI, or nonfatal stroke. In the LEADER trial, the GLP-1 RA liraglutide significantly reduced the primary outcome, largely driven by improvements in CV-related death and all-cause mortality (19). In the SUSTAIN-6 trial, semaglutide significantly improved the primary outcome, an effect that was largely driven by reduction of non-fatal stroke events (20). In both the trials, there were numerically fewer non-fatal MI events (19,20). Results from these trials suggest that the CV benefit of GLP-1 RAs in T2DM patients was driven by improvements in vascular risk factors and atherosclerosis rather than improving HF. GLP-1 RAs have extra-pancreatic actions on the CV system which are probably responsible for the improvements noted in CV risk factors like weight, BP, and lipid parameters. Although the DPP-4 inhibitors extend the actions of endogenous incretins, they appear to have diverse actions on other inflammatory and metabolic pathways which could have negated the CV protective role of prolonging GLP-1 activity. However, DPP-4 inhibitors were proven to be safe from a CV standpoint based on the available recent data despite concerns regarding increased HF risk prompting the issue of FDA warnings (30,32,33,36-38).
In the EMPA-REG OUTCOME trial, empagliflozin, with its renal-specific actions, dramatically reduced the risk of CV deaths, HF hospitalizations, and all-cause mortality with RR reductions of 30–40% in these outcomes, prompting cardiologists to prescribe this medication to T2DM patients at risk for HF (46). Significant improvement in primary outcome from this trial was largely driven by reduced mortality and HF hospitalization rate rather than prevention of vascular/atherosclerotic events. The reduced HF hospitalizations could be from the diuretic action and BP lowering effect of empagliflozin (53). This mechanism of action, along with proven benefit in a large randomized trial, can make empagliflozin an important component of T2DM therapy in patients with HF with reduced ejection fraction. Empagliflozin caused slight improvements in other CV risk factors like weight, BP, and lipid profiles (46). Interestingly, in this trial, improvement in the primary outcome and HF hospitalizations was apparent very early in the course of the trial at around 3 months from randomization and persisted throughout the duration of the study (46). Improvements in mortality occurred early and improved across the 3-year study period (46). Also important are the nephroprotective actions of SGLT-2 inhibitors as observed with canagliflozin and empagliflozin. Nephroprotection is important in patients with CV disease and HF as worsening kidney function can mutually worsen HF. Further larger studies which are currently undergoing will provide clarity on the nephroprotective actions of SGLT-2 inhibitors (40). Given the possibility of differential improvements in CV disease from GLP-1 agonists and SGLT-2 inhibitors, future research should include trials to evaluate the beneficial effects of the combination of these drugs to improve atherosclerotic and HF outcomes (Figure 1).
Conclusions
T2DM is growing in pandemic proportions and is likely to affect a large proportion of adult population worldwide in the coming years. Being associated with significant morbidity, mortality, and health care expenditures, it is imperative to control the responsible risk factors. Metabolic and CV risks pose a major threat in the T2DM population accounting for its major healthcare implications. Emerging evidence suggest that in T2DM patients, hyperglycemia plays a little role in the progression of CV disease, and metabolic risk factors like insulin resistance, hypertension, obesity, and dyslipidemia are the major culprits in the initiation and progression of CV disease. Hence it is imperative to adopt a holistic risk factor control approach when managing a T2DM patient. Interestingly, newer anti-hyperglycemic medications like the GLP-1 RAs and SGLT-2 inhibitors showed promise in recent clinical trials in terms of providing CV benefit irrespective of the glycemic control. However, these findings were largely apparent in patients with established CV disease as they were more represented in the randomized controlled trials evaluating these drugs. Currently undergoing larger trials will provide more information and clarity on the CV protective role of these newer medications. Studies evaluating the CV protective role of the newer T2DM medications in patients without established CV disease are necessary to establish the role of these drugs in the treatment paradigm of T2DM. As T2DM or insulin resistance syndrome, CV disease, and HF are frequently coexistent, it would be interesting to design studies evaluating the combinations of GLP-1 RAs, SGLT-2 inhibitors, and pioglitazone in T2DM patient at an elevated CV risk, and in non-diabetic patients with insulin resistance to study the possible CV protective role of these combinations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta. Available online: https://www.cdc.gov/diabetes/data/statistics/2014StatisticsReport.html. Accessed 27 January 2017.
- International Diabetes Federation. Available online: http://www.idf.org/about-diabetes/facts-figures. Accessed 27 January 2017.
- Emerging Risk Factors Collaboration, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215-22. [Crossref] [PubMed]
- Emerging Risk Factors Collaboration, Di Angelantonio E, Kaptoge S, et al. Association of cardiometabolic multimorbidity with mortality. JAMA 2015;314:52-60. [Crossref] [PubMed]
- Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141:421-31. [Crossref] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53. [Crossref] [PubMed]
- Patel A, MacMahon S, Chalmers J, et al. The ADVANCE collaborative group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560-72. [Crossref] [PubMed]
- Gerstein HC, Miller ME, Byington RP, et al. The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545-59. [Crossref] [PubMed]
- Obunai K, Jani S, Dangas GD. Cardiovascular morbidity and mortality of the metabolic syndrome. Med Clin North Am 2007;91:1169-84. [Crossref] [PubMed]
- Towfighi A, Ovbiagele B. Metabolic syndrome and stroke. Curr Diab Rep 2008;8:37-41. [Crossref] [PubMed]
- Pawlyk AC, Giacomini KM, McKeon C, et al. Metformin pharmacogenomics: current status and future directions. Diabetes 2014;63:2590-9. [Crossref] [PubMed]
- Dormandy JA, Charbonnel B, Eckland DJ, et al. PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomized controlled trial. Lancet 2005;366:1279-89. [Crossref] [PubMed]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457-71. [Crossref] [PubMed]
- Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, 2008 Guidance for industry: diabetes mellitus — evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. Available online: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf. Accessed: 27 January 2017.
- European Medicine Agency, Committee for Medicinal Products for Human Use, 2010 Guideline on clinical investigation of medicinal products in the treatment of diabetes mellitus. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/02/WC500073570.pdf. Accessed: 27 January 2017.
- Kim W, Egan J. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 2008;60:470-512. [Crossref] [PubMed]
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab 2015;6:19-28. [Crossref] [PubMed]
- Ratner R, Han J, Nicewarner D, et al. Cardiovascular safety of exenatide BID: an integrated analysis from controlled clinical trials in participants with type 2 diabetes. Cardiovasc Diabetol 2011;10:22. [Crossref] [PubMed]
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular Outcomes in type 2 diabetes. N Engl J Med 2016;375:311-22. [Crossref] [PubMed]
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834-44. [Crossref] [PubMed]
- Lorber D. GLP-1 receptor agonists: effects on cardiovascular risk reduction. Cardiovasc Ther 2013;31:238-49. [Crossref] [PubMed]
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696-705. [Crossref] [PubMed]
- Nathanson D, Ullman B, Lofstrom U, et al. Effects of intravenous exenatide in type 2 diabetic patients with congestive heart failure: a double-blind, randomised controlled clinical trial of efficacy and safety. Diabetologia 2012;55:926-35. [Crossref] [PubMed]
- Katout M, Zhu H, Rutsky J, et al. Effect of GLP-1 mimetics on blood pressure and relationship to weight loss and glycemia lowering: results of a systematic meta-analysis and meta-regression. Am J Hypertens 2014;27:130-9. [Crossref] [PubMed]
- Sun F, Wu S, Wang J, et al. Effect of glucagonlike peptide-1 receptor agonists on lipid profiles among type 2 diabetes: a systematic review and network meta-analysis. Clin Ther 2015;37:225-41.e8. [Crossref] [PubMed]
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247-57. [Crossref] [PubMed]
- Koska J, Sands M, Burciu C, et al. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes. Diab Vasc Dis Res 2015;12:154-63. [Crossref] [PubMed]
- Barbieri M, Rizzo MR, Marfella R, et al. Decreased carotid atherosclerotic process by control of daily acute glucose fluctuations in diabetic patients treated by DPP-IV inhibitors. Atherosclerosis 2013;227:349-54. [Crossref] [PubMed]
- Monami M, Lamanna C, Desideri CM, et al. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther 2012;29:14-25. [Crossref] [PubMed]
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317-26. [Crossref] [PubMed]
- Kongwatcharapong J, Dilokthornsakul P, Nathisuwan S, et al. Effect of dipeptidyl peptidase-4 inhibitors on heart failure: A meta-analysis of randomized clinical trials. Int J Cardiol 2016;211:88-95. [Crossref] [PubMed]
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327-35. [Crossref] [PubMed]
- White WB. Cardiovascular outcomes with alogliptin in patients with type 2 diabetes mellitus and recent acute coronary syndromes. European Association for the Study of Diabetes. Barcelona, 23-27 September 2013.
- Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385:2067-76. [Crossref] [PubMed]
- White WB, Kupfer S, Zannad F, et al. Cardiovascular mortality in patients with type 2 diabetes and recent acute coronary syndromes from the EXAMINE trial. Diabetes Care 2016;39:1267-73. [Crossref] [PubMed]
- Green JB, Bethel MA, Armstrong PW, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2015;373:232-42. [Crossref] [PubMed]
- Rosenstock J, Marx N, Neubacher D, et al. Cardiovascular safety of linagliptin in type 2 diabetes: a comprehensive patient-level pooled analysis of prospectively adjudicated cardiovascular events. Cardiovasc Diabetol 2015;14:57. [Crossref] [PubMed]
- Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med 2016;374:1145-54. [Crossref] [PubMed]
- Diabetes Medications Containing Saxagliptin and Alogliptin: Drug Safety Communication - Risk of Heart Failure. Available online: https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm494252.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery. Accessed: 20 May 2017.
- Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015;12:90-100. [Crossref] [PubMed]
- Triggle CR, Ding H. Cardiovascular impact of drugs used in the treatment of diabetes. Ther Adv Chronic Dis 2014;5:245-68. [Crossref] [PubMed]
- Kumar R, Kerins DM, Walther T. Cardiovascular safety of anti-diabetic drugs. Eur Heart J Cardiovasc Pharmacother 2016;2:32-43. [Crossref] [PubMed]
- Baker WL, Smyth LR, Riche DM, et al. Effects of sodium- glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens 2014;8:262-75.e9. [Crossref] [PubMed]
- Oliva RV, Bakris GL. Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors. J Am Soc Hypertens 2014;8:330-9. [Crossref] [PubMed]
- Bode B, Stenlöf K, Harris S, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes Metab 2015;17:294-303. [Crossref] [PubMed]
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117-28. [Crossref] [PubMed]
- Heerspink HJ, Desai M, Jardine M, et al. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 2017;28:368-75. [Crossref] [PubMed]
- Wanner C, Inzucchi JM, Lachin D, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323-34. [Crossref] [PubMed]
- CANVAS. Available online: clinicaltrials.gov. Identifier: NCT01032629.
- DECLARE-TIMI58. Available online: clinicaltrials.gov. Identifier: NCT01730534.
- Cardiovascular Outcomes Following Treatment With Ertugliflozin in Participants With Type 2 Diabetes Mellitus and Established Vascular Disease. Available online: clinicaltrials.gov. Identifier: NCT01986881.
- Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;374:1321-31. [Crossref] [PubMed]
- Abdul-Ghani M, Prato SD, Chilton R, et al. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. Diabetes Care 2016;39:717-25. [Crossref] [PubMed]


