A review of the management of complex para-pneumonic effusion in adults
Introduction
The pleural sac is the space between the lung (lined by visceral pleura) and the chest wall (lined by the parietal pleura). This space normally contains a thin layer of fluid called the pleural fluid, which functions as a lubricant and assists in reducing friction between the two lining surfaces during respiration. The pleural fluid is produced by the capillaries in parietal pleura and is absorbed by the lymphatics in the parietal pleura, which can under normal circumstances absorb the fluid at rate of about 20 times the rate of production (1). Under certain disease states, the fluid can also enter the pleural cavity from the lung interstitium via the visceral pleura or from the communications across the diaphragm to the peritoneal space (2). A pleural effusion (PE) is said to be present when there is excessive fluid within the pleural space and occurs when the rate of accumulation of the fluid exceeds the rate of drainage. Patients suspected of having PE are evaluated further typically by imaging of the chest like a chest roentgenogram or ultrasound or CT scan to further characterize the effusion. Once the presence of PE is established, the next step to acquire and analyze the pleural fluid (3). Broadly, the effusions are classified as either transudative or exudative (4). Transudative effusions are caused by systemic factors like congestive heart failure (CHF) or nephrotic syndrome which alter the hydrostatic and/or oncotic forces across the capillaries involved in formation or removal of the pleural fluid (5). Exudative effusions on the other hand are usually caused by local factors like inflammation of pleura or the lungs. Other mechanisms of exudative effusions include impaired lymphatic drainage of the pleural fluid, disruption of thoracic duct and movement of peritoneal fluid through connections across the diaphragm, etc. (6). The most widely used criteria for differentiation of transudative and exudative effusions are the Light’s criteria which as follows (4).
- Pleural fluid protein/serum protein ratio greater than 0.5;
- Pleural fluid lactate dehydrogenase (LDH)/serum LDH ratio greater than 0.6;
- Pleural fluid LDH level greater than two-thirds the upper limit of the laboratory’s reference range of serum LDH.
The effusion is considered to be exudative when one of the three criteria is met. If none of the criteria are met, the effusion is considered to be transudative. Transudative effusions usually respond to medical treatment of the systemic factors contributing to the effusion. However, the exudative effusions can pose a diagnostic and therapeutic challenge, often needing non-surgical/surgical intervention(s) in addition to medical management (6,7).
Overview of management of exudative effusions
The first step in the management of a new onset PE is a diagnostic thoracentesis or tap (6). Only in few specific circumstances is a tap not warranted, for example if there is a strong suspicion of heart failure as a cause of the effusion(s), then a trial of medical therapy for heart failure can be done prior to a thoracentesis (8). The differentiation between transudative and exudative effusions is made based on the analysis of pleural fluid as explained above. Certain characteristics of the pleural fluid increase the possibility of the effusion being complicated, which include: loculated fluid, pH <7.2, glucose <60 mg/dL, positive gram stain or culture and gross purulence. In the presence of these factors or if the fluid re-accumulates after initial tap, a repeat thoracentesis should be performed (9). If the fluid can’t be completely removed, then there are various options available including tube thoracostomy, use of intrapleural fibrinolytics, video assisted thoracoscopic surgery (VATS) and open drainage (OD) (10). Discussing the utility of these various procedures is the crux of this review.
Management of parapneumonic effusions
Parapneumonic pleural effusion (PPE) refers to a PE associated with a focus of infection in the lungs, which can be bacterial pneumonia, a pulmonary abscess, or infected bronchiectasis (11). It is seen in about 20% of the hospitalized patients with pneumonia (12) and approximately 1 million patients develop PPEs annually in the United States (US) (13). Empyema is said to be present when there is gross purulence or presence of bacteria in the pleural fluid as evidenced by gram stain. It is divided into early (stage I or exudative) and advanced stages (stage II or fibrinopurulent; and stage III or organizing). Also, the presence of thickened parietal pleura on contrast enhanced CT scan is suggestive of empyema (14-16). The rate of parapneumonic empyema related hospitalizations in the US have doubled from 1996 to 2008 and the trend was observed across all age groups (17). Empyema is associated with substantial cost burden and remains an important cause of morbidity and mortality. Early diagnosis, appropriate antimicrobial therapy and adequate drainage of effusion are important in decreasing morbidity and mortality (18). Antibiotic therapy must cover the bacteria implicated in causing pneumonia in that particular setting. Also, anaerobic coverage must be added if there is clinical suspicion or microbiological evidence of involvement of anaerobic bacteria in the infection. The decision to drain the PPE is made based on three important considerations: pleural space anatomy, pleural fluid chemistry and pleural fluid microbiology. A panel developed by the Health and Science Policy Committee of the American College of Chest Physicians classified PPE into four groups based on risk for poor outcome, which is presented in Table 1 at the end of the article (25).
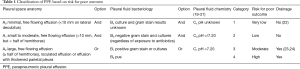
Full table
Based on the consensus opinion, the panel recommended that patients with category 1 and category 2 risk for poor outcome with PPE may not require drainage; however, drainage was recommended for management of category 3 and 4 PPE. Therapeutic thoracentesis or tube thoracostomy alone may be insufficient treatment for managing most patients with category 3 or 4 PPE. Fibrinolytics, VATS, and surgery are acceptable approaches for managing patients with category 3 and category 4 PPE which do not respond to less invasive drainage methods.
The panel however exercised caution while interpreting the above recommendations as some of the studies reviewed has revealed conflicting data (26,27).
Though antibiotic therapy alone may be sufficient in certain cases, it remains to be ascertained whether early fluid drainage of complicated effusions has better outcomes compared to conservative therapy alone in such cases (28,29). Regardless, antibiotic therapy is mandatory in all cases of PPE. The following approach is reasonable based on the risk category:
- Category 1 PPE: medical treatment alone;
- Category 2 PPE: pleural fluid sampling (via thoracentesis) must be done, with drainage of all free flowing fluid, if a small bore catheter is used;
- Category 3 and 4 PPE: tube thoracostomy must be performed and clinical status reviewed in 24 hours. If there is incomplete drainage, then intrapleural fibrinolytics must be instituted. After this, if there is incomplete resolution at 72 hours, then surgical drainage is warranted.
Tube thoracostomy
Tube thoracostomy or chest tube drainage is the least invasive method of draining the pleural fluid after therapeutic thoracentesis. For free flowing fluid which is uniloculated, chest tube drainage usually works well. However, this form of drainage is also frequently used, at least as the initial approach in the management of multiloculated effusions as well. While multiloculated effusions are typically drained using smaller bore catheters to allow placement of multiple tubes, the uniloculated effusions were traditionally drained using large bore catheters to allow thick, viscous fluid to be drained. However, with increasing evidence suggesting that smaller tubes are not associated with inferior outcomes, large bore tubes are falling out of favor. The Multi-center Intrapleural Streptokinase Trial (MIST 1) concluded that there was no significant difference in mortality or need for thoracic surgery among large (15–20 F), medium (10–14 F), or small (<10 F) tubes (30). Also, patients on whom large bore tubes were used complained of significantly higher level of pain. Chest tube insertions for complicated effusions must be done under imaging guidance as the added cost for the procedure is expected to be more than compensated by the increased success of drainage (31). Some studies have shown that performer skills in chest tube insertion may be better in those who undergo training on cadavers and medical simulators compared to the conventional methods of training (32,33). Regular flushes can help maintain the patency of small bore catheters, especially when the fluid is thick. A post procedure CT scan within 24 hrs is needed to confirm proper placement. Chest tubes are usually left in place until the daily output from the tube falls below 50 mL.
Thoracoscopic debridement
VATS allows for a safe, effective and relatively less invasive means of drainage of pleural fluid and has the advantage of being able to be converted to thoracotomy if adequate drainage is not achieved. Both debridement and decortication can be performed through VATS. It has also been considered as the “gold standard” diagnostic procedure for investigation of pleural exudates. In one study, empyema fluid culture positivity with VATS (84.6%) was significantly higher than pre-VATS fluid culture (35%). While chest tube drainage alone may be sufficient for early stage empyema, the more advanced stages have a high mortality rate without more advanced methods of drainage. For example, one study evaluated outcomes for different procedures in advanced empyema (defined as stage II or more). The success rate (no death, no additional drainage procedures) with VATS (around 80%) was double the success rate of tube thoracostomy (40%) and was comparable with that of thoracotomy (89%) (34).
Common indications for VATS drainage include multiloculated empyema, empyema refractory to tube thoracostomy and presence of pleural peel (35). In one retrospective study, the success rate for drainage of early stage multiloculated empyema was 66%, 95% and 100% in the tube thoracostomy, fibrinolytic therapy and VATS groups, respectively (36). The relatively high success rate and lack of significant morbidity makes VATS a preferred method of drainage. Though the safety and benefit of VATS have been documented beyond doubt in multiple studies, there are no evidence based recommendations on the precise stage and timing for VATS. VATS has revolutionized the management of empyema in the last decade and its role has progressed from a diagnostic purpose to an intermediate procedure for empyemas refractory to medical therapy and as an early drainage procedure for complicated empyemas in order to spare invasive surgery. There is data suggesting improved outcomes with VATS as a primary drainage method compared to chest tube drainage at no significant cost difference. More studies are needed to further establish the benefit of VATS as a primary drainage procedure, especially from the cost perspective and there is a possibility of a paradigm shift in the future in the initial drainage approach for empyema (37).
VATS decortication (VATSD) was initially evaluated as a treatment option for early (stage I) empyema. However, recently, several studies have suggested superior outcomes with VATSD compared to Open Decortication (OD) even in advanced stage II and stage III empyema. Still, there is some concern expressed about the ability of VATSD to achieve complete decortication in advanced empyema. In the near future, VATSD can be expected to evolve as the preferred approach for management of even advanced empyema compared to OD.
Intrapleural fibrinolytics and DeoxyriboNuclease (DNase)
There is conflicting evidence and opinion about the use of fibrinolytics alone to assist in the drainage of complicated effusions. The earlier supposition that intrapleural fibrinolytics improved outcomes, which was based on small trials and case series (38-42) was refuted by a meta-analysis (43) and the MIST 1 trial (44). A Cochrane review of 7 studies recruiting 761 patients did not show any difference in mortality with the use of fibrinolytics. However, it was found that the use of fibrinolytics decreased the need for surgical intervention, except that such a benefit was not shown in the newer MIST 1 trial that was included in the review. A more recent double blinded randomized cross over study showed that intra pleural alteplase (25 mg) was associated with less treatment failure (which was defined as <50% improvement of effusion on CT scan) compared to placebo. The dose range of intrapleural TPA is reported to be between 10 and 100 mg daily with most patients needing 3 to 4 doses (41). Adverse reactions with intra-pleural fibrinolytics include pain, fever and allergic reactions. Intrapleural fibrinolysis is not associated with systemic thrombolysis and one study showed that pleural hemorrhage occurred only in those who were on systemic anticoagulation when receiving intrapleural fibrinolytics.
The main component contributing to viscosity of exudative fluid is DNA which led to the use of intrapleural DNase in the management of complicated effusions. The MIST 2 trial which was a double blind randomized study concluded that Intrapleural t-PA-DNase therapy improved fluid drainage in patients with pleural infection and reduced the frequency of surgical referral and the duration of the hospital stay (45). Treatment with DNase alone or t-PA alone was concluded to be ineffective. Another multinational observation study looked at the outcomes in 107 patients who received intrapleural tPA/DNase. Treatment success at 30 days, volume of pleural fluid drained, improvement in radiographic pleural opacity and inflammatory markers, need for surgery, and adverse events were the measured outcomes. Over 90% of the patients were successfully managed with intrapleural tPA/DNase and did not need surgical intervention. Majority of the patients received tPA/DNase more than 24 hours after failing antibiotics and tube thoracostomy. Fluid drained increased from a median of 250 mL in the 24 hours preceding commencement of intrapleural therapy to 2,475 mL in the 72 hours following treatment initiation (P<0.05). There was a corresponding decrease in pleural opacity and C-reactive protein. Adverse events included pain and non-fatal bleeding. With these recent studies, it is reasonable to conclude that intrapleural tPA/DNase is a safe and effective drainage option (unlike tPA alone which had conflicting evidence) especially as a rescue therapy for patients who do not respond to initial conservative management with antibiotics and chest tube drainage (46). While, it is clear that tPA/DNAase is helpful, the exact dosing may still need to be determined so as to have maximal beneficence and minimal complications.
OD
Advanced stages of empyema are characterized by deposition of fibrin on the pleural surfaces and formation of “pleural peel”, which can limit re-expansion of the lung leading to significant symptoms. In such late stages, decortication/total pleurectomy is usually needed to allow the re-expansion of the lung. The invasive option is usually reserved for patients whose exercise tolerance and quality of life remain limited for over six months due to the fibrotic changes in the pleura. Though OD was traditionally used for these advanced empyemas, new evidence suggests better outcomes with VATSD, which is taking over OD as the initial procedure of choice for such empyemas (47). One study analyzed the predicting factors for conversion thoracotomy and OD following VATS. Factors studied included age, sex, presence of bacteria, presence of gram-positive and gram-negative organisms, and time interval between onset of symptoms and surgery, as well as different etiologic groups (postpneumonic, postoperative, posttraumatic, postembolic, tuberculosis). It was found that delayed referral (>2 weeks) and presence of gram-negative microorganisms were the only significant predictors for conversion thoracotomy in a multivariate analysis (48).
Conclusions
Management of complex pleural effusion remains ever changing. VATS debridement if fibrinolytics fail seems to have the best outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Light RW. Disorders of the pleura. Harrison’s principles of Internal Medicine. 19th edition. McGraw Hill, 2015:1716-9.
- Porcel JM, Light RW. Diagnostic approach to pleural effusion in adults. Am Fam Physician 2006;73:1211-20. [PubMed]
- Saguil A, Wyrick K, Hallgren J. Diagnostic approach to pleural effusion. Am Fam Physician 2014;90:99-104. [PubMed]
- Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972;77:507-13. [Crossref] [PubMed]
- Chetty KG. Transudative pleural effusions. Clin Chest Med 1985;6:49-54. [PubMed]
- Sahn SA. The differential diagnosis of pleural effusions. West J Med 1982;137:99-108. [PubMed]
- Light RW. Diagnostic principles in pleural disease. Eur Respir J 1997;10:476-81. [Crossref] [PubMed]
- Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002;346:1971-7. [Crossref] [PubMed]
- Light RW, Rodriguez RM. Management of parapneumonic effusions. Clin Chest Med 1998;19:373-82. [Crossref] [PubMed]
- Na MJ, Dikensoy O, Light RW. New trends in the diagnosis and treatment in parapneumonic effusion and empyema. Tuberk Toraks 2008;56:113-20. [PubMed]
- Ferreiro L, San José ME, Valdés L. Management of Parapneumonic Pleural Effusion in Adults. Arch Bronconeumol 2015;51:637-46. [PubMed]
- Porcel JM, Light RW. Parapneumonic pleural effusions and empyema in adults: current practice. Rev Clin Esp 2009;209:485-94. [Crossref] [PubMed]
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis 2007;45:1480-6. [Crossref] [PubMed]
- Waite RJ, Carbonneau RJ, Balikian JP, et al. Parietal pleural changes in empyema: appearances at CT. Radiology 1990;175:145-50. [Crossref] [PubMed]
- Aquino SL, Webb WR, Gushiken BJ. Pleural exudates and transudates: diagnosis with contrast-enhanced CT. Radiology 1994;192:803-8. [Crossref] [PubMed]
- Kirsch E, Gückel C, Kaim A, et al. The findings and value of computed tomography in pleural empyema. Rofo 1994;161:404-11. [Crossref] [PubMed]
- Grijalva CG, Zhu Y, Nuorti JP, et al. Emergence of parapneumonic empyema in the USA. Thorax 2011;66:663-8. [Crossref] [PubMed]
- Ashbaugh DG. Empyema thoracis. Factors influencing morbidity and mortality. Chest 1991;99:1162-5. [Crossref] [PubMed]
- Heffner JE, Brown LK, Barbieri C, et al. Pleural fluid chemical analysis in parapneumonic effusions. A meta-analysis. Am J Respir Crit Care Med 1995;151:1700-8. [Crossref] [PubMed]
- Lesho EP, Roth BJ. Is pH paper an acceptable, low-cost alternative to the blood gas analyzer for determining pleural fluid pH? Chest 1997;112:1291-2. [Crossref] [PubMed]
- Cheng DS, Rodriguez RM, Rogers J, et al. Comparison of pleural fluid pH values obtained using blood gas machine, pH meter, and pH indicator strip. Chest 1998;114:1368-72. [Crossref] [PubMed]
- Light RW, Girard WM, Jenkinson SG, et al. Parapneumonic effusions. Am J Med 1980;69:507-12. [Crossref] [PubMed]
- Ferguson AD, Prescott RJ, Selkon JB, et al. The clinical course and management of thoracic empyema. QJM 1996;89:285-9. [Crossref] [PubMed]
- Himelman RB, Callen PW. The prognostic value of loculations in parapneumonic pleural effusions. Chest 1986;90:852-6. [Crossref] [PubMed]
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000;118:1158-71. [Crossref] [PubMed]
- Wait MA, Sharma S, Hohn J, et al. A randomized trial of empyema therapy. Chest 1997;111:1548-51. [Crossref] [PubMed]
- Angelillo Mackinlay TA, Lyons GA, Chimondeguy DJ, et al. VATS debridement versus thoracotomy in the treatment of loculated postpneumonia empyema. Ann Thorac Surg 1996;61:1626-30. [Crossref] [PubMed]
- Berger HA, Morganroth ML. Immediate drainage is not required for all patients with complicated parapneumonic effusions. Chest 1990;97:731-5. [Crossref] [PubMed]
- Lim TK. Management of parapneumonic pleural effusion. Curr Opin Pulm Med 2001;7:193-7. [Crossref] [PubMed]
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010;137:536-43. [Crossref] [PubMed]
- Moulton JS. Image-guided management of complicated pleural fluid collections. Radiol Clin North Am 2000;38:345-74. [Crossref] [PubMed]
- Chung TN, Kim SW, You JS, et al. Tube thoracostomy training with a medical simulator is associated with faster, more successful performance of the procedure. Clin Exp Emerg Med 2016;3:16-19. [Crossref] [PubMed]
- Takayesu JK, Peak D, Stearns D. Cadaver-based training is superior to simulation training for cricothyrotomy and tube thoracostomy. Intern Emerg Med 2017;12:99-102. [Crossref] [PubMed]
- Wozniak CJ, Paull DE, Moezzi JE, et al. Choice of first intervention is related to outcomes in the management of empyema. Ann Thorac Surg 2009;87:1525-30. [Crossref] [PubMed]
- Luh SP, Chou MC, Wang LS, et al. Video-assisted thoracoscopic surgery in the treatment of complicated parapneumonic effusions or empyemas: outcome of 234 patients. Chest 2005;127:1427-32. [PubMed]
- Metin M, Yeginsu A, Sayar A, et al. Treatment of multiloculated empyema thoracis using minimally invasive methods. Singapore Med J 2010;51:242-6. [PubMed]
- Zahid I, Nagendran M, Routledge T, et al. Comparison of video-assisted thoracoscopic surgery and open surgery in the management of primary empyema. Curr Opin Pulm Med 2011;17:255-9. [Crossref] [PubMed]
- Davies RJ, Traill ZC, Gleeson FV. Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection. Thorax 1997;52:416-21. [Crossref] [PubMed]
- Bouros D, Schiza S, Tzanakis N, et al. Intrapleural urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. A randomized, double-blind study. Am J Respir Crit Care Med 1999;159:37-42. [Crossref] [PubMed]
- Diacon AH, Theron J, Schuurmans MM, et al. Intrapleural streptokinase for empyema and complicated parapneumonic effusions. Am J Respir Crit Care Med 2004;170:49-53. [Crossref] [PubMed]
- Thommi G, Nair CK, Aronow WS, et al. Efficacy and safety of intrapleural instillation of alteplase in the management of complicated pleural effusion or empyema. Am J Ther 2007;14:341-5. [Crossref] [PubMed]
- Tuncozgur B, Ustunsoy H, Sivrikoz MC, et al. Intrapleural urokinase in the management of parapneumonic empyema: a randomised controlled trial. Int J Clin Pract 2001;55:658-60. [PubMed]
- Tokuda Y, Matsushima D, Stein GH, et al. Intrapleural fibrinolytic agents for empyema and complicated parapneumonic effusions: a meta-analysis. Chest 2006;129:783-90. [Crossref] [PubMed]
- Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005;352:865-74. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014;11:1419-25. [Crossref] [PubMed]
- Shahin Y, Duffy J, Beggs D, et al. Surgical management of primary empyema of the pleural cavity: outcome of 81 patients. Interact Cardiovasc Thorac Surg 2010;10:565-7. [Crossref] [PubMed]
- Lardinois D, Gock M, Pezzetta E, et al. Delayed referral and gram-negative organisms increase the conversion thoracotomy rate in patients undergoing video-assisted thoracoscopic surgery for empyema. Ann Thorac Surg 2005;79:1851-6. [Crossref] [PubMed]


