Quantitative computed tomography to predict postoperative FEV1 after lung cancer surgery
Introduction
Surgical resection is the recommended treatment for patients with non-metastatic non-small cell lung cancer (NSCLC). One of the main limitations of surgical treatment is the predicted postoperative lung function. Several methods can be used to more accurately estimate postoperative respiratory function, such as diffusing capacity of the lungs for carbon monoxide (DLCO) and VO2 max. However, the most commonly used and most widely recommended functional test is estimation of the predicted postoperative FEV1 (ppoFEV1) (1). Loss of pulmonary function depends on the extent of the surgical procedure as well as the quality of the remaining lung parenchyma. Precise ppoFEV1 estimation is essential and largely recommended in patients with COPD with preoperative FEV1 less than to 80% predicted (2). At the present time, ppoFEV1 estimation in these patients is usually performed using algorithmic formula, or morphologic examination using 19 segments ventilation/perfusion scintigraphy (2,3). This 19 segments ventilation/perfusion scintigraphy allow the estimation of ppoFEV1 taking account of the lung parenchyma functionality, but represent an expensive and highly irradiating examination, and may be difficult to interpret in a context of chronic and/or acute pulmonary disease. Chest computed tomography (CT)-scan constitutes part of the morphological assessment during preoperative staging of NSCLC, and appears to be relevant to estimate ppoFEV1 based on 3D reconstructions of lung parenchyma and volumetric estimations.
The primary objective of this study was to evaluate quantitative CT for estimation of ppoFEV1, and to compare quantitative CT to other methods of ppoFEV1 estimation. These estimates were compared to FEV1 measured 3 months post-operatively (poFEV1) after NSCLC surgical resection.
Methods
Patients
We performed a prospective, single center study on 120 patients, over 12 months between January 2013 and January 2014 in our Thoracic Surgery Department at Amiens University Hospital. Eligible patients had to have non-metastatic NSCLC or suspected lung cancer, after a thoracic oncology multidisciplinary meeting had proposed surgical resection as the best curative treatment option. Each patient included underwent preoperative assessment of pulmonary function by spirometry, a 19 segment ventilation/perfusion scintigraphy, quantitative CT, and spirometry 3 months postoperatively. Patients with CT performed in another hospital were excluded, in order to obtain similar CT acquisition parameters and standardized reconstruction of lung parenchyma during volumetry using the same dedicated software. Patients with incomplete preoperative lung function evaluation and patients presenting factors likely to influence the 3-month poFEV1 such as atelectasis, pleural effusion, pneumothorax, or pneumonia were also excluded.
Procedure
Preoperative pulmonary function tests were performed in each patient to estimate ppoFEV1. ppoFEV1 was estimated by 5 different modalities: 2 algorithmic methods based on preoperative spirometry, 2 methods based on 19 segment ventilation/perfusion scintigraphy, and quantitative CT.
Algorithmic methods based on preoperative spirometry were the Nakahara formula: ppoFEV1 = [1 − (n − a) / (42 − a)] × FEV1, with [n] the number of resected sub segments in the lobe or lung and [a] the number of sub segments obstructed by tumor (4); and the Juhl & Frost formula: ppoFEV1 = FEV1 × (1 − S × 0.0526) with [S] the number of resected segments out of 19 pulmonary segments (5).
Methods based on 19 segment ventilation/perfusion scintigraphy used separately ventilation data and perfusion data following the formula: ppoFEV1 = FEV1 × (1 – FC), where [FC] is the functional contribution of the lung parenchyma to be resected (6). This preoperative lung function assessment is applied in the French Epithor database.
Finally, ppoFEV1 was estimated by quantitative CT approach, based on contrast-enhanced chest CT performed during preoperative morphologic staging assessment of NSCLC. Quantitative CT was performed in our center according to a standardized procedure: 64 channel MDCT (GE Healthcare, Waukesha, WI, USA®) with contrast material injection (IOMERON 350 ®) using a mechanical injector at a rate of 1.5 mL/s, for a total dose of 100 mL using a slice thickness of 0.6 mm reconstructed with 0.6 mm intervals, 70 seconds after injection. Reconstruction was performed on a dedicated workstation: advantage window 4.6 (GE Healthcare, Waukesha, WI, USA) using its Thoracic VCAR quantitative measurement function with multiplanar reconstruction (MPR), volume-rendering technique (VRT), and 3D reconstruction of the lungs, allowing calculation of the lung parenchyma volume. This assessment was performed twice and separately by 2 radiologists, to avoid any error during the calculation procedure. Tumor volume including the lobe or lung to be resected and emphysema lesions defined by a −950 HU limit were subtracted from the total lung volume in order to estimate the ppoFEV1 according to the formula: ppoFEV1 = FEV1 × (1-V), where [V] is tumor volume and emphysema lesions (Figure 1). These 5 ppoFEV1 assessment methods were compared to the 3-month postoperative poFEV1 evaluated by spirometry.
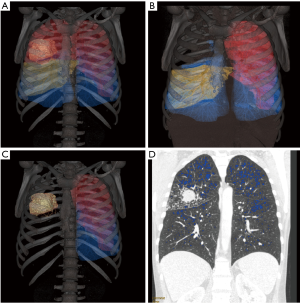
Statistical analysis
These 5 methods of ppoFEV1 estimation were compared to the 3-month poFEV1 using Pearson’s correlation coefficient. FEV1, ppoFE1, poFEV1 and the mean volume difference between ppoFEV1 and poFEV1 are expressed as mean ± standard deviation for each method.
Results
Among the 120 patients with a non-metastatic NSCLC eligible for surgery, 23 were included during the 12 months study’s period. Seventy-one patients for whom chest CT was performed in another center, 12 patients for whom preoperative 19 segment ventilation/perfusion scintigraphy was not performed, 12 patients presenting factors likely to influence the 3-month postoperative poFEV1 such as atelectasis (5 cases), pleural effusion or pneumothorax (3 cases), or pneumonia (4 cases), 1 death and 1 patient lost to follow-up, were excluded. Patient characteristics are resumed in Table 1.
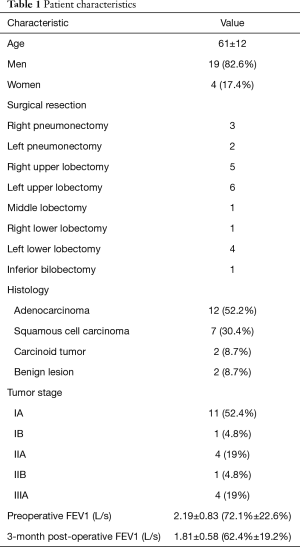
Full table
ppoFEV1 and mean volume difference with poFEV1 were calculated for each method of assessment; with a ppoFEV1 of 1.61±0.73 L/s and a mean difference of 320±262 mL for Nakahara formula; ppoFEV1 of 1.60±0.72 L/s and a mean difference of 332±251 mL for Juhl & Frost formula; ppoFEV1 of 1.70±0.71 L/s and a mean difference of 312±303 mL for 19 segment ventilation scintigraphy; ppoFEV1 of 1.72±0.72 L/s and a mean difference of 304±295 mL for 19 segment perfusion scintigraphy; and ppoFEV1 =1.65±0.68 L/s and a mean difference of 266±229 mL for quantitative CT, Table 2.
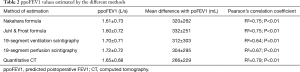
Full table
According to Pearson’s correlation coefficient, the best correlation with 3-month poFEV1 was obtained with quantitative CT ppoFEV1 estimation, with R2=0.79 (P<0.01). Algorithmic estimation formulas had an R2=0.75 for Nakahara (P<0.01) and R2=0.75 for Juhl & Frost (P<0.01) while 19 segment scintigraphy had an, R2=0.67 for perfusion scintigraphy (P<0.01) and an R2=0.64 for ventilation scintigraphy (P<0.01).
Discussion
Several studies have evaluated the feasibility and accuracy of quantitative CT estimation of ppoFEV1, confirming the validity of this assessment technique (7-10), which is more readily available than magnetic resonance imaging, which was not evaluated in this study (9-12). Wu et al. compared a quantitative CT estimation of ppoFEV1 with perfusion scintigraphy estimation of ppoFEV1 for 44 patients undergoing surgical anatomical resection of NSCLC including 28 pneumonectomies and 16 lobectomies, with R=0.88 vs. R=0.86 for pneumonectomies, and R=0.90 vs. R=0.80 for lobectomies, respectively (7). Liu et al. studied a series of 31 patients with 2 pneumonectomies, 23 lobectomies, 3 segmentectomies, 1 lobectomy associated with segmentectomy and 2 non-anatomical resections, and compared the quantitative CT estimation of ppoFEV1 with poFEV1 measured between 1 and 6 months postoperatively, with a mean volume difference of 190 mL (10 to 720 mL) (8). A more powerful study by Ohno et al., including 229 patients with multimodal estimation of ppoFEV1 with quantitative CT, magnetic resonance imaging, and PET scan found a correlation with poFEV1 with a mean difference of 4.7%±14.2%, 4.4%±14.2% and 5.1%±14.7%, respectively (10). The present study confirms the results of these studies and shows that quantitative CT is a relevant approach to estimation of ppoFEV1 and can be easily used in clinical practice in patient with early stage NSCLC, in order to assess the possibility of pulmonary parenchyma resection with precise estimation of postoperative respiratory function.
This is an inexpensive technique, as quantitative CT volumetry is based on the CT scan performed as part of preoperative staging of NSCLC. Three-dimension reconstruction and evaluation of tumor volume or emphysema lesions can be rapidly assessed at a multidisciplinary meeting, without any major extra work required.
The main benefit of this method is that quantitative CT does not require any irradiating investigation in addition to the staging CT scan and appears to be less invasive than 19-segment ventilation/perfusion scintigraphy. Moreover, this method calculates the volume of lung parenchyma to be resected, but also subtract the emphysema lesions in remaining lung parenchyma, which is not assessed with other methods of estimation.
Limitation of this study is the sample size, with 23 patients evaluated. Also, this quantitative CT still does not resolve the problem of evaluation of pulmonary function in patients with interstitial lung disease, pleural effusion or pneumothorax, which can alter quantitative CT calculation of ppoFEV1.
The persistent volume differences between quantitative CT estimation of ppoFEV1 and poFEV1 measured 3 months postoperatively could possibly be explained by the patient’s position during the examination, as the patient is in the supine position during CT scan, causing ventilatory disturbances in the lower lobes and modification of diaphragm kinetics due to intra-abdominal pressure. These phenomena are not observed during spirometry in the standing position. Furthermore, these volume differences must be interpreted in the light of the variability between 2 spirometries performed in the same patient, which Miller et al. have estimated to be 106±100 mL (13).
In conclusion, quantitative CT appears to be a satisfactory method for estimation of ppoFEV1, and appears to be more relevant than other approaches. Performed as part of preoperative staging, this estimation does not involve any additional morphologic examinations, particularly irradiating 19-segment ventilation/perfusion scintigraphy. We propose the use of quantitative CT as the reference method for estimation of ppoFEV1 in patients with a preoperative FEV1 less than 80% predicted.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our institutional review board and patients were included in our indexed database under the serial DRCI T38 after written informed consent was obtained from the patient.
References
- Wyser C, Stulz P, Solèr M, et al. Prospective evaluation of an algorithm for the functional assessment of lung resection candidates. Am J Respir Crit Care Med 1999;159:1450-6. [Crossref] [PubMed]
- Colice GL, Shafazand S, Griffin JP, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:161S-77S.
- Wernly JA, DeMeester TR, Kirchner PT, et al. Clinical value of quantitative ventilation-perfusion lung scans in the surgical management of bronchogenic carcinoma. J Thorac Cardiovasc Surg 1980;80:535-43. [PubMed]
- Nakahara K, Monden Y, Ohno K, et al. A method for predicting postoperative lung function and its relation to postoperative complications in patients with lung cancer. Ann Thorac Surg 1985;39:260-5. [Crossref] [PubMed]
- Juhl B, Frost N. A comparison between measured and calculated changes in the lung function after operation for pulmonary cancer. Acta Anaesthesiol Scand Suppl 1975;57:39-45. [Crossref] [PubMed]
- Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 1989;139:902-10. [Crossref] [PubMed]
- Wu MT, Pan HB, Chiang AA, et al. Prediction of postoperative lung function in patients with lung cancer: comparison of quantitative CT with perfusion scintigraphy. AJR Am J Roentgenol 2002;178:667-72. [Crossref] [PubMed]
- Liu F, Han P, Feng GS, et al. Using quantitative CT to predict postoperative pulmonary function in patients with lung cancer. Chin Med J (Engl) 2005;118:742-6. [PubMed]
- Ohno Y, Koyama H, Nogami M, et al. Postoperative lung function in lung cancer patients: comparative analysis of predictive capability of MRI, CT, and SPECT. AJR Am J Roentgenol 2007;189:400-8. [Crossref] [PubMed]
- Ohno Y, Koyama H, Nogami M, et al. State-of-the-art radiological techniques improve the assessment of postoperative lung function in patients with non-small cell lung cancer. Eur J Radiol 2011;77:97-104. [Crossref] [PubMed]
- Iwasawa T, Saito K, Ogawa N, et al. Prediction of postoperative pulmonary function using perfusion magnetic resonance imaging of the lung. J Magn Reson Imaging 2002;15:685-92. [Crossref] [PubMed]
- Ohno Y, Hatabu H, Higashino T, et al. Dynamic perfusion MRI versus perfusion scintigraphy: prediction of postoperative lung function in patients with lung cancer. AJR Am J Roentgenol 2004;182:73-8. [Crossref] [PubMed]
- Herpel LB, Kanner RE, Lee SM, et al. Variability of Spirometry in Chronic Obstructive Pulmonary Disease, Results from two clinical trials. Am J Respir Crit Care Med 2006;173:1106-13. [Crossref] [PubMed]


