Electro-physiological evidence of intercostal nerve injury after thoracotomy: an experimental study in a sheep model
Introduction
Thoracotomy is the most widely used approach in thoracic surgery (1). One of the common sequelae following thoracotomy is post-thoracotomy pain syndrome (PTPS), which occurs in 25–60% of cases and leads to deteriorated lung function, delayed recovery and reduced quality of life (2-5). Though PTPS is a fairly complex multifactorial condition, it is believed that intercostal nerve injury due to stretch and ischemia is the most important pathological factor (3,5). So far, very few studies have investigated the mechanism of intercostal nerve injury caused by thoracotomy in detail (3). In a unique study of Rogers et al. intercostal nerves were stimulated and motor evoked potentials were recorded from intercostal muscles in 13 patients undergoing thoracotomy (4). They found a total conduction block in the nerve immediately above the thoracotomy incision in all patients after the rib retractor was removed. Nevertheless, many questions remain open, such as: when is the first occurrence of nerve conduction block during thoracotomy and how long does it persist after removal of the retractor?
In the last two decades, various surgical techniques have been developed and practiced to avoid intercostal nerve damage caused by thoracotomy. In a clinical study involving 280 consecutive patients undergoing elective thoracotomy for pulmonary resection, Dr. Cerfolio and co-workers demonstrated that intercostal sutures were associated with less PTPS than conventional pericostal sutures (6). Furthermore, another prospective randomized study found that harvesting and then leaving the intercostal muscle flap dangled between the retractor blades (dangling muscle flap technique) resulted in further reduced PTPS, when added to intercostal sutures (7). However, this finding could not be confirmed by the prospective, randomized study of Wu et al. (8). These contradictory results again highlight the importance of a profound understanding of intercostal nerve injury for the further improvement of surgical techniques and development of innovative instruments to reduce PTPS.
Therefore, we opted to evaluate the electro-physiological changes indicative of intercostal nerve injury on a large-animal model of lateral thoracotomy.
Methods
We obtained approval from the local Committee for Animal Care (Thüringer Landesamt für Verbraucherschutz, Registration No. 08-003/13) and conducted experiments in three adult sheep (Leineschaf, W&P Agrarhandels GmbH, Oberheldrungen, Germany) weighing around 80 kg in adherence with guidelines on animal experimentation.
After premedication with Azaperon (2 mg/kg, i.m.) and Atropine (0.05 mg/kg, i.m.) general anesthesia was induced with 10% Ketamine (0.2 mL/kg, i.v.) and 2% Xylazine (0.1 mL/kg, i.v.) and maintained with 2.5% isoflurane/oxygen mix, Fentanil (5–10 µg/kg every 20–40 minutes i.v.) and Midazolam (0.05 mg/kg, i.v.). All surgical procedures were performed under endotracheal intubation and mechanical ventilation (Siemens servo 900c ventilator, Siemens-Elema, Solna, Sweden) with an inspirational oxygen fraction of 50%. Lactated Ringer’s solution was infused at a rate of 20 mL/kg/h. Muscular relaxant was not used.
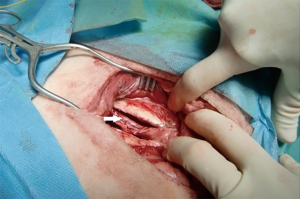
In the left lateral decubitus a 10 cm skin incision was made at the sixth intercostal space, followed by division of underlying connective tissue and exploration. Thoracotomy in the sixth intercostal space was performed by employing diathermy to the superior border of the seventh rib. The ribs were then spread using a Finochietto retractor for a distance of seven cm for 30 minutes according to conventional thoracotomy in two study sheep. In the third sheep, the thoracotomy was followed by harvesting intercostal muscles including the neurovascular bundle adjacent to the inferior edge of the sixth rib using a periosteal elevator (Figure 1). Thereafter, the ribs were spread in the same way, but with the muscle flap dangled between the blades for intercostal nerve protection (dangling muscle flap technique).
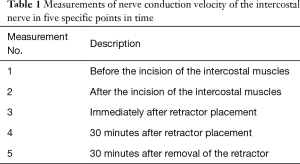
The nerve conduction velocity of the intercostal nerve was recorded by means of Nicolet Viking IV P (Nicolet, Madison, WI, USA) in five specific points in time (Table 1). Taking into account the duration of intubation anesthesia, measurements thereafter were not considered in the present experiment. Prior to thoracotomy, bipolar recording needle electrodes were placed in the anterior border of the intercostal muscle, a ground electrode in the serratus anterior muscle, a stimulating reference needle electrode in the erector spinae muscle. The stimulus given by a stimulating probe on the inferior border of the sixth rib was increased from 3.5 mA with 300 µs pulse width until the maximal stimulus included response was achieved. Repeat measurements (n=3) of the nerve conduction velocity were recorded after delivery of a supramaximal stimulus of 8.0 mA for three times at intervals of 2 minutes for each measurement. The nerve conduction velocity was calculated by means of the distance between stimulating probe and recording electrodes and the latency between stimulus and muscle evoked potentials. The results are presented as mean ± standard deviation.
Full table
After surgery the sheep were monitored every day for any adverse events. Buprenorphin (10 µg/kg s.c., three times daily) and Carprofen (4 mg/kg s.c., once daily) were given during the first 3 post-operative days. In the following 2 days Carprofen (4 mg/kg s.c., once daily) combined with Novaminsulfon (1 g) in the drinking water were administered, if sheep appeared to experience pain. Thereafter, sheep received Novaminsulfon (1 g) as oral analgesic if necessary.
Results
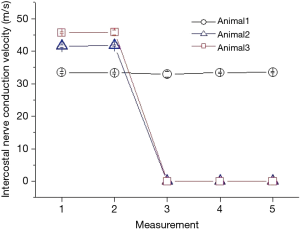
All thoracotomies and chest closures were completed successfully. All sheep survived the surgery without encountering adverse events. The changes in intercostal nerve conductions derived from three sheep are demonstrated in Figure 2. No deterioration in nerve conduction was found before and after incision of the intercostal muscles (measurement 1 and 2) in all three sheep. The stimulation induced intercostal nerve conduction velocities were 33.45±0.35, 41.56±0.35 and 45.63±0.37 before incision and 33.33±0.29, 41.73±0.12 and 45.76±0.08 after incision in sheep 1, 2 and 3 respectively. Statistically no differences in the nerve conduction velocity were observed before and after incision in each sheep. In the two sheep undergoing conventional thoracotomy and rib spreading, the physiological conductivity of the intercostal nerve was completely blocked immediately after retractor placement using the same stimulation intensity or even the supra-threshold intensity (measurement 3). The conduction block persisted 30 minutes after retractor placement and a further 30 minutes after removal (measurements 4 and 5). In contrast, the nerve conduction was not impaired throughout the examination in the sheep undergoing dangling muscle flap technique.
Discussion
Thoracotomy, along with limb amputation, is considered to be the procedure that elicits the highest risk of severe chronic postoperative pain (5,9). In the development of PTPS, type A delta nerve fiber-mediated somatic pain produced by the direct injury to the thoracic wall and the sensitization of the central nervous system play an important role (5,10). However, the neuropathic pain caused by intercostal nerve injury is believed to be the most important pathologic factor (4,5,9). The intercostal nerves belong to the somatic nervous systems and are composed of dorsal horn sensory afferent fibers, ventral horn motor efferent fibers, and postganglionic sympathetic nerves. Electromyography and nerve-conduction studies are well-established neurophysiologic techniques used to assess the integrity of larger myelinated sensory and motor fibers. Clinical studies have suggested that both sensory and motor nerves injuries may contribute to the development of PTPS (10-13).
In the present study, we consciously chose a sheep model, as it closely reflects human anatomic, and physiologic characteristics (14). We performed conventional thoracotomy and rib spreading and measured the electro-physiological changes of the intercostal nerve compressed by the retractor. This was compared to the electro-physiological changes in the context of dangling muscle flap thoracotomy, which does not cause intercostal nerve compression. Our results demonstrate that opening the intercostal space by employing electrocautery does not impair the function of intercostal nerve. In addition, the nerve conduction block occurred immediately after rib spreading with the rib retractor and persisted throughout the rib spreading. These findings are in accordance with the previous report in the study of Roger et al., showing a total conduction block in the nerve immediately above the incision after removal of rib retractor in all patients (4). As another important finding of the present experiment, we measured the nerve conduction velocity 30 minutes after removal of rib retractor, and found that nerve conduction block persisted at least for 30 minutes after release of mechanical compression. Considering the intact nerve conduction in the sheep undergoing dangling muscle flap technique, our results indicate that intercostal nerve injury is primarily attributed to the mechanical compression caused by the rib retractor. Furthermore, rib spreading for 30 minutes might result in permanent injury to the intercostal nerve. Indeed, animal experiments with rats have demonstrated that rib retraction for 60 minutes following thoracotomy produced allodynia and extensive axon loss in the intercostal nerves of the retracted ribs (15). Given that the chest cavity is opened for more than 30 minutes in most of the thoracic surgical procedures, our experiment highlights again that the protection of the intercostal nerve is crucial to reduce the incidence of PTPS. Based on our results, we believe that dangling muscle flap technique should be preferred, when thoracotomy and rib spreading are necessary. Moreover, considering the vulnerability of intercostal nerve to conventional rib retractor, our findings also underscore the importance of developing novel technologies for rib retraction. In fact, experimental studies in sheep have shown that force-controlled retraction by means of a novel instrumented retractor was correlated with reduced tissue damage and animal stress (16).
As an alternative approach to thoracotomy, video-assisted thoracoscopic (VATS) lobectomy has been increasingly adopted for treatment of early-stage NSCLC and is associated with reduced postoperative complications, equal oncological efficacy and long-term outcomes in comparison to open lobectomy (17,18). In this minimally invasive approach, anatomic lobectomy and mediastinal lymph node dissection or sampling are performed using one, two or three ports without retractor use or rib spreading (19). Various prospective clinical trials have demonstrated that the VATS approach results in reduced postoperative pain and allows a rapid functional recovery of patients (20,21). Dr. Miyazaki and colleagues assessed intercostal nerve function by means of current perception threshold testing in 32 patients undergoing either VATS, video-assisted minithoracotomy with metal retractors or conventional thoracotomy (12). Neither changes in current perception threshold values nor residual pain more than 12 weeks after surgery were found in the VATS group. In contrast, the video-assisted mini-thoracotomy with metal retractors group and the conventional thoracotomy group were associated with significantly higher current perception threshold values at 2,000 Hz 1 week after surgery and pain in approximately 70% of patients 12 weeks after surgery. Our results mirrored these findings and support the assertion that performing VATS when possible could achieve a faster recovery and improve the perioperative quality of life.
The authors do recognize various limitations of the present experiment. We present preliminary results regarding the electro-physiological changes of intercostal nerve after thoracotomy and rib spreading. Of concern is also the very low number of animals that have been included. However, all five different electro-physiological measurement were repeated three times for each sheep which shows homogenous parameters for each measurement. In addition, we only recorded the nerve conduction velocity of intercostal nerve up to 30 minutes after removal of the retractor. In these contexts, future electro-physiological measurements in more animals and in an extended time course after removal of the retractor will be conducted for better understanding the intercostal nerve injury caused by thoracotomy and rib spreading.
In conclusion, our animal experiment provided further electro-physiological evidence on intercostal nerve injury after thoracotomy and rib spreading. Intercostal nerve injury is primarily attributed to the mechanical compression caused by the rib retractor. The functional impairment persists after release of the compression.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors obtained approval from the local Committee for Animal Care (Thüringer Landesamt für Verbraucherschutz, Registration No. 08-003/13) and conducted experiments in adherence with guidelines on animal experimentation.
References
- Hall MJ, DeFrances CJ, Williams SN, et al. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report 2010.1-20, 24. [PubMed]
- Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus 2004;16:E1. [Crossref] [PubMed]
- Rogers ML, Duffy JP. Surgical aspects of chronic post-thoracotomy pain. Eur J Cardiothorac Surg 2000;18:711-6. [Crossref] [PubMed]
- Rogers ML, Henderson L, Mahajan RP, et al. Preliminary findings in the neurophysiological assessment of intercostal nerve injury during thoracotomy. Eur J Cardiothorac Surg 2002;21:298-301. [Crossref] [PubMed]
- Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg 2009;36:170-80. [Crossref] [PubMed]
- Cerfolio RJ, Price TN, Bryant AS, et al. Intracostal sutures decrease the pain of thoracotomy. Ann Thorac Surg 2003;76:407-11; discussion 411-2. [Crossref]
- Cerfolio RJ, Bryant AS, Maniscalco LM. A nondivided intercostal muscle flap further reduces pain of thoracotomy: a prospective randomized trial. Ann Thorac Surg 2008;85:1901-6; discussion 1906-7.
- Wu N, Yan S, Wang X, et al. A prospective, single-blind randomised study on the effect of intercostal nerve protection on early post-thoracotomy pain relief. Eur J Cardiothorac Surg 2010;37:840-5. [Crossref] [PubMed]
- Benedetti F, Amanzio M, Casadio C, et al. Postoperative Pain and Superficial Abdominal Reflexes After Posterolateral Thoracotomy. Ann Thorac Surg 1997;64:207-10. [Crossref] [PubMed]
- Liu XG, Pang RP, Zhou LJ, et al. Neuropathic Pain: Sensory Nerve Injury or Motor Nerve Injury? Adv Exp Med Biol 2016;904:59-75. [Crossref] [PubMed]
- Jänig W, Levine JD, Michaelis M. Interactions of sympathetic and primary afferent neurons following nerve injury and tissue trauma. Prog Brain Res 1996;113:161-84. [Crossref] [PubMed]
- Miyazaki T, Sakai T, Tsuchiya T, et al. Assessment and follow-up of intercostal nerve damage after video-assisted thoracic surgery. Eur J Cardiothorac Surg 2011;39:1033-9. [Crossref] [PubMed]
- Yang Y, Dai L, Ke M. Spontaneous muscle contraction with extreme pain after thoracotomy treated by pulsed radiofrequency. Pain Physician 2015;18:E245-9. [PubMed]
- Martini L, Fini M, Giavaresi G, et al. Sheep model in orthopedic research: a literature review. Comp Med 2001;51:292-9. [PubMed]
- Buvanendran A, Kroin JS, Kerns JM, et al. Characterization of a new animal model for evaluation of persistent postthoracotomy pain. Anesth Analg 2004;99:1453-60. [Crossref] [PubMed]
- Bolotin G, Buckner GD, Jardine NJ, et al. A novel instrumented retractor to monitor tissue-disruptive forces during lateral thoracotomy. J Thorac Cardiovasc Surg 2007;133:949-54. [Crossref] [PubMed]
- Burt BM, Kosinski AS, Shrager JB, et al. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg 2014;148:19-28. [Crossref] [PubMed]
- Su S, Scott WJ, Allen MS, et al. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J Thorac Cardiovasc Surg 2014;147:747-52. [Crossref] [PubMed]
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]
- Andreetti C, Menna C, Ibrahim M, et al. Postoperative pain control: videothoracoscopic versus conservative mini-thoracotomic approach. Eur J Cardiothorac Surg 2014;46:907-12. [Crossref] [PubMed]
- Palade E, Guenter J, Kirschbaum A, et al. Postoperative pain in the acute phase after surgery: VATS lobectomy vs. open lung resection - results of a prospective randomised trial. Zentralbl Chir 2014;139:S59-66. [PubMed]


