Inflammation, phagocytosis and cancer: another step in the CD47 act
Breast cancer is the most common cancer in women, with an estimated 1.7 million new diagnoses per year (1). Depending on the mode of immunohistochemical or molecular classification used, subdivision into four [luminal A, luminal B, Her2-enriched, basal-like (2)] or up to ten (3) molecular subtypes has been reported. Risk factors for development of breast cancer include reproductive and hormonal characteristics as early menarche and late menopause, hormone replacement therapy, nulliparity and advanced age at first pregnancy, with the last three factors correlating with molecular subtypes (4).
The pathogenesis of breast cancer is heterogeneous, with genomic instability leading to accumulation of activating as well as loss-of-function mutations that drive tumour progression (5). Aberrations within a tumour cell may be located on the genomic, transcriptional, translational, or protein level and largely depends on the molecular subtype.
CD47 molecule is a transmembrane protein expressed by many human cell types. One of its functions involves the role as a ligand for the signal regulatory protein-α (SIRPα) that is expressed by dendritic cells and macrophages (6). Haematopoietic stem cells, for example, express CD47 to protect themselves from phagocytosis when passing through bony sinusoids filled with phagocytes (7). As soon as having reached their marrow niches, CD47 expression on stem cells starts to decrease again (7). One survival mechanism of cancer cells is achieved by overexpressing CD47 on their surface, thus defending themselves against phagocytosis from macrophages. Though the notion that cancer cells frequently up-regulate CD47 compared to their normal counterparts is now widely accepted, the underlying up- and down-stream regulatory mechanisms are not fully elucidated. Previous studies including breast cancer identified that the promoter region for the CD47 gene is regulated by binding of several transcription factors, including MYC and NF-κB (8,9). However, the exact mechanism behind the overexpression of CD47 in cancer cells is insufficiently understood (10).
In a recent study published in Nature Communications by Betancur et al., a CD47-associated specific regulatory genomic locus, a so-called “super-enhancer” (SE), was identified (10). In general, a SE constitutes a gene region with strong enrichment of transcriptional co-activators as mediator (MED1) (11). Typically, an accumulation of H3K27ac, a modification of the DNA packaging protein H3, is also present and indicates regions of active chromatin (10). Any of these factors may be used to define a SE that usually covers an area of >20 kilobases (kb), thus measuring a multitude of a “normal” enhancer (11). SEs are regarded as a specific feature gained by tumour cells, since they are absent in normal tissue (10). They comprise several clusters of normal enhancers, the so-called constituent enhancers (10). Correspondent to their function, SEs are preferentially located near oncogenes or gene regions rearranged by translocation (12).
So far, Betancur et al. could identify SEs within 200 kb of the CD47 gene region in the diffuse large B-cell lymphoma (DLBCL) cell-line LY4 and the T-cell acute lymphoblastic leukaemia (T-ALL) cell-lines RPMI18402, MOLT3 and Jurkat (10). Moreover, the authors discovered that the breast cancer cell-lines HCC1954 (HER2 positive) and MCF7 (luminal A) have SEs down-stream of the CD47 gene (10). Interestingly, those cell lines with CD47-associated SEs strongly overexpress CD47, whilst cells without identifiable SEs express CD47 at significantly lower levels. Moreover, the location of the SEs varies depending on the tumour type; whilst SEs upstream of CD47 are found in DLBCL and T-ALL, the SE regulating CD47 in breast cancer cell-lines is located downstream of the gene (10). However, presence of SEs also varies between breast cancer subtypes. According to Betancur et al., the human epidermal growth factor (Her2) positive cell line HCC1954 as well as the oestrogen receptor (ER) and progesterone receptor (PR) positive cell line MCF have SEs associated with CD47, whilst SEs are absent in triple-negative (ER−, PR−, Her2−) breast cancer samples (10). Within the CD47-associated SE, the constituent enhancer E5 has an enhancing effect on CD47 transcription in the ER+ and PR+ cell-line MCF7 (10).
In order to understand the regulation of SEs better, Betancur et al. identified transcription factors that bind to the E5 constituent enhancer and thus potentially regulate CD47 transcription upstream, namely NFAT, NF-κB, STAT 3/5/6, SMAD and PPAR. However, whilst knockdown of STAT3, STAT5 and STAT6 with short hairpin RNAs (shRNAs) does not significantly alter expression of CD47, the protein expression is reduced when NF-κB1 and PPARα are knocked down (10).
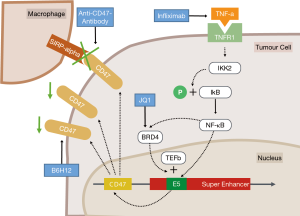
According to subsequent in vitro analyses in the study by Betancur et al., the reduction of NF-κB1 results in increased phagocytosis of tumour cells by macrophages, ultimately resulting in reduction of tumour volume in vivo (10). A similar effect is achieved by using an antibody blocking the interaction between SIRPα on macrophages and CD47 on tumour cells (Figure 1) (13). By combining NF-κB1 knockdown with a CD47-blocking antibody, the rate of phagocytosis significantly increases (10).
NF-κB1 recruits the bromodomain-containing protein 4 (BRD4) to these hyperacetylated chromatin regions (i.e., SEs) in tumour cells (14). After binding to the SEs, BRD4 promotes gene transcription by linking the enhancers to the transcription elongation factor (TEFb) complex (14). Consequently, in the study by Betancur et al. the use of the BRD4-inhibitor JQ1 likewise decreases CD47 expression levels in breast cancer cells (Figure 1) (10).
Another way how breast cancer cells increase CD47 expression is via the TNF inflammatory pathway, which is an upstream activation step of NF-κB1 (15). Related to this, Betancur et al. could show that activation of the TNF pathway via stimulation of cell-line MCF7 with TNF-α significantly increases CD47 expression (10). This effect is achieved by activation of the inhibitor of nuclear factor kappa-B kinase subunit beta (IKK2) that phosphorylates the inhibitor of NF-κB-complex (IkB), thus enabling passage of NF-κB into the nucleus (Figure 1) (15). On the other hand, the authors demonstrated that the blockage of the TNF inflammatory pathway by using infliximab was associated with increased phagocytosis of breast cancer cells by macrophages (10). Thus, combining TNF-alpha blockers with anti-CD47 treatment strategies might be a valuable therapeutic approach for further clinical trials.
Notably, CD47 does not only play a role in breast cancer, but has a prognostic and therapeutic significance in other malignancies. Elevated expression of CD47 constitutes a poor prognostic factor in acute myeloid leukaemia (AML) stem cells (16). By directly blocking CD47 with anti-CD47 monoclonal antibodies, phagocytosis of AML stem cells is reinforced (16).
In hepatocellular carcinoma (HCC), overexpression of CD47 on the cellular surface is associated with resistance to the small molecule-inhibitor sorafenib (9). Again, NF-κB is involved in the upstream regulation of CD47 expression. Consequently, NF-κB is activated in HCC resistant to sorafenib. Moreover, NF-κB and CD47 levels positively correlate between each other in human-derived HCC samples (9).
Moreover, CD47 is overexpressed in about half of patient-derived gastric cancer samples and up to 90% of gastric cancer cell-lines (17). Tumours with high CD47 levels tend to grow faster and form spheroid colonies in vitro. Direct blockage of CD47 with B6H12 results in enhanced phagocytosis of tumour cells (Figure 1) (17). Moreover, high levels of CD47 constitute a poor prognostic factor in patients with gastric cancer (17).
According to Betancur et al., the overexpression of CD47 is one mechanism of cancer cells to escape phagocytosis by macrophages via bondage to the SIRPα (10). CD47-overexpression is not only associated with drug resistance (9), but also constitutes a poor prognostic factor in clinical practice (17).
CD47 is regulated by various constituent enhancers located in SEs that have cancer type-specific positions. In breast cancer, NFKB1 binds directly to the E5 constituent enhancer, thus promoting CD47 transcription. By disrupting the TNF-inflammatory pathway upstream of NF-κB1, CD47-expression is significantly reduced (10).
In clinical practice, targeting of the TNF inflammatory pathway or its downstream effectors NF-κB or BDR4 may reduce tumour progression by enhancing phagocytosis through macrophages located in the tumour microenvironment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [Crossref] [PubMed]
- Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012;486:346-52. [PubMed]
- Turkoz FP, Solak M, Petekkaya I, et al. Association between common risk factors and molecular subtypes in breast cancer patients. Breast 2013;22:344-50. [Crossref] [PubMed]
- Luen S, Virassamy B, Savas P, et al. The genomic landscape of breast cancer and its interaction with host immunity. Breast 2016;29:241-50. [Crossref] [PubMed]
- Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem 1999;274:559-62. [Crossref] [PubMed]
- Jaiswal S, Jamieson CH, Pang WW, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009;138:271-85. [Crossref] [PubMed]
- Casey SC, Tong L, Li Y, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016;352:227-31. [Crossref] [PubMed]
- Lo J, Lau EY, Ching RH, et al. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology 2015;62:534-45. [Crossref] [PubMed]
- Betancur PA, Abraham BJ, Yiu YY, et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun 2017;8:14802. [Crossref] [PubMed]
- Whyte WA, Orlando DA, Hnisz D, et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 2013;153:307-19. [Crossref] [PubMed]
- Pott S, Lieb JD. What are super-enhancers? Nat Genet 2015;47:8-12. [Crossref] [PubMed]
- Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012;109:6662-7. [Crossref] [PubMed]
- Chapuy B, McKeown MR, Lin CY, et al. Discovery and characterization of super-enhancer-associated dependencies in diffuse large B cell lymphoma. Cancer Cell 2013;24:777-90. [Crossref] [PubMed]
- Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer 2013;12:86. [Crossref] [PubMed]
- Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009;138:286-99. [Crossref] [PubMed]
- Yoshida K, Tsujimoto H, Matsumura K, et al. CD47 is an adverse prognostic factor and a therapeutic target in gastric cancer. Cancer Med 2015;4:1322-33. [Crossref] [PubMed]