The role of video-assisted thoracoscopic surgery for management of symptomatic pleural effusion after coronary artery bypass surgery: a best evidence topic report
Clinical scenario
A 57-year-old man was referred to your attention for the management of a left-sided symptomatic pleural effusion (PE). He underwent coronary artery bypass graft (CABG) surgery with a left internal thoracic artery (LITA) graft for unstable angina three months earlier. During follow-up, he had received two thoracentesis for symptomatic left-sided PE. Despite clinical improvements, each time the effusion re-accumulated, leading to a recrudescence of the dyspnoea. In both circumstances, the effusion fluid appeared serous with lymphocyte predominance but without neoplastic cells. How should this patient be managed and treated? Should we apply chest drainage and performing through that a blind talc pleurodesis (talc slurry), leaving him at risk of pleural adhesions formation or we should directly perform a video-assisted thoracoscopic surgery (VATS) talc pleurodesis in order to reduce the risk of PE recurrence and of trapped lung?
Why is this question important?
PE is very common clinical entity after CABG and can be detected in approximately 90% of patients during the first week of operation. Most are small volumes, left-sided effusions that resolve without intervention. Occasionally, the persistence of PE or the development of new effusions within the first postoperative months represents one of the main causes of hospital readmission. These effusions can be broadly classified into four phenotypes by the time at which the effusion occurs after operation: (I) perioperative (within the first week); (II) early (within the first months); late (2 to 12 months); and persistent (after 6 months) (1). The choice of a treatment rather than another depends on the PE pathogenesis and time of occurrence. The perioperative and early effusions are associated with acute chest pain and fever and usually require anti-inflammatory medication. The late and persistent PEs are the consequence of dysfunctional healing of the pleural space. For these types of effusion, the best treatment remains unknown and unexplored, including repeated thoracentesis, chest drainage, thoracoscopic talc pleurodesis or decortication in case of trapped lung. Thus, the clinical hypothesis of this study is whether performing VATS pleurodesis rather than talc slurry through chest drainage is justified in patients with symptomatic PEs after CABG that do not resolve following repeated thoracentesis. The rationale of this strategy is to prevent trapped lung and to avoid more aggressive surgical procedures as decortication. In the next section, we systematically review the literature to obtain the state-of-the-art evidence to support our hypothesis.
Who will benefit from VATS?
Patients with recurrent, symptomatic PEs after CABG not responding to repeated thoracentesis can be safely and effectively treated with VATS. PEs occurring in the immediate postoperative time are usually small. Thoracentesis is rarely performed; the management is conservative and effusion usually resolves within two weeks without clinical sequel. Diagnostic and therapeutic thoracentesis should be performed in patients with large, symptomatic PE. If chest pain, fever, and lymphocytic exudates on pleural fluid analysis are also present, these patients likely present a post cardiac injury syndrome (PCIS) and corticosteroids after initial thoracentesis should be administered (1). Patients with PEs unresponsive to repeated thoracentesis should directly undergo VATS talc pleurodesis rather than other procedures as chest drainage to make pleural cavity free from adhesions and avoid further more invasive treatment as thoracotomy decortication due to trapped lung.
Search strategy and study selection
Electronic database of PubMed was searched from inception to August 2016 using OVID interface [pleural effusion] AND [CABG.mp OR exp coronary artery by-pass graft surgery/] AND [VATS.mp OR exp. Video-assisted Thoracoscopic Surgery/]. Finally, a hand search was used to follow-up references from the retrieved studies. Seventy-six papers were found using the reported research strategy. Studies that met the following criteria were considered eligible: (I) prospective, retrospective, and observational studies investigating the treatment of PE after CABG; (II) studies conducted in adult patients. Exclusion criteria were (I) abstracts; (II) case reports; (III) reviews and commentaries; (IV) non-English language papers; (V) papers reporting causes of PE different from CABG. Finally, 10 papers (2-11), summarized in Table S1, were identified that provided the most applicable evidence to answer the question.
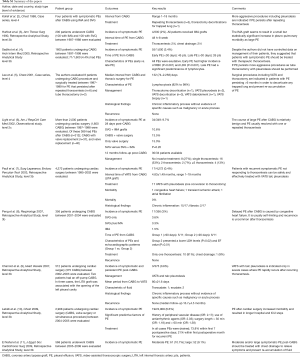
Full table
Results
The present review included two case-series study including ≥4 patients (2,5), 7 retrospective analytical studies (3,4,7-11), and one observational study (6) but no randomized controlled trials (RCTs). The score of the level of evidence according to the criteria of Centre for Evidence Based Medicine (CEBM) (12) was low. In fact, only one study presented a level of evidence of 2C (6), 7 studies a level of 3b (3,4,7-11), and two studies (2,5) a level of 4.
Kollef et al. (2) reported four patients with delayed and symptomatic PE post-CABG. Three patients responded to ≥1 thoracentesis, while one patient underwent thoracotomy decortication for trapped lung. Hurlbut et al. (3) found 4/200 patients with symptomatic post-CABG PE. Two responded to thoracentesis and 2 to tube thoracostomy. Sadikot et al. (4) evaluated 71 post-CABG PEs. All PE were exudative. Patients with early PE (n=45) had higher incidence of red blood cells (P<0.001) and of lactate dehydrogenase (LDH) (P<0.001) within fluid, while patients with late PE (n=26) a significant predominance of lymphocytes. Lee et al. (5) followed 8 patients with PE that persisted for two years after CABG. VATS (n=3) and thoracotomy decortication (n=1) were performed in 4/8 cases for trapped lung. In the other 4 patients, VATS pleurodesis was applied for non-loculated effusions. In all cases no recurrence occurred and histological findings showed chronic inflammatory process without malignancy or acute disease. Light et al. (6) evaluated more than 2,000 patients undergoing cardiac surgery. Of these, 389 had PEs after CABG (n=312), after CABG with valve replacement (n=37), and after only valve replacement (n=40). Incidence of symptomatic PE at 28 days post-CABG was 9.7% (34/349). Similar incidence was found between patients who received SVG plus IMA grafts (10.6%), patients who received CABG with valve surgery (13.5%), and patients who received only valve surgery (15.0%). No significant difference was found between patients who received only SVG versus those receiving SVG with IMA (P=0.20). They followed for 12 months 30/34 patients with symptomatic post-CABG PE. Eight patients (12%) were treated with medical treatment only, 16 (53%) with a single therapeutic thoracentesis, and 4 (13%) with ≥3 thoracentesis. One patient was still receiving periodic thoracentesis 12 months post-CABG. Paull et al. (7) found 17/4,272 (0.4%) patients having PE after cardiac surgery refractory to thoracentesis. All but one of these were successfully treated with VATS pleurodesis. In one case a conversion to thoracotomy was performed. Morbidity and mortality rates were 17.6% and 0%, respectively, with the most common morbidity being congestive heart failure, transient ischemic attack, and atrial fibrillation. Peng et al. (8) found 11/356 (3%) symptomatic post-CABG PEs. Late PEs (n=6) compared to early PE (n=5) had lower levels of LDH and lower ejection fraction, supporting congestive heart failure as aetiology. The incidence of PE was similar between patients who received SVG only (3.6%); patients who received SVG plus IMA (3.3%), and patients who received IMA graft only (1.5%). Thoracentesis successfully resolved PEs in 10/11 cases. Charniot et al. (9) reported on 3 patients with symptomatic post CABG PE, recurred after 3–5 thoracentesis and treated with VATS and talc pleurodesis. No recurrence was noted. Histological findings of pleural biopsies showed chronic inflammatory disease alone. Labidi et al. (10) found 12/2,908 patients with early symptomatic PE after cardiac surgical procedures including CABG. Chest drainage treated 73.8% of PE that occurred within the first week and 21% of those that presented within first month. El Nahal et al. (11) retrospectively reviewed 568 patients undergoing CABG. Of these 61 (10.7%) had moderate and 52 (9.1%) large PE. Thoracentesis (38/61 moderate PE) and chest thoracostomy (52/52 large PE) were performed to relieve symptoms.
Discussion
Post-CABG PE was categorized by Heidecker (1) into four categories depending on interval time of occurrence: perioperative (within first week), early (within first month), late (2 to 12 months), and persistent (after 6 months). Perioperative PEs are common and usually resolve without any intervention. Persistent and delayed PE can be associated with significant morbidity and hospital readmission. Their rate may be under-reported in the literature due to not rigorously defined diagnostic criteria and/or follow up of these patients with multiple doctors, including cardiothoracic surgeons, cardiologist, pulmonologists, and internists, all of whom may have different management strategies. The rate of symptomatic post-CABG PEs ranged between 2–9.7%. In the most of the papers (2,3,5-11) different grafts were used. The incidence of early PE is generally higher with use of IMA grafts compared to SVC grafts since the necessity to enter to adjacent pleura space while mobilizing and harvesting the IMA. However, two papers (6,8) found no-significant difference on the incidence of late PE between patients who received SVG plus IMA grafts, patients who received CABG with valve surgery, and patients who received only valve surgery (6,8) and between patient who received only SVG versus SVG with IMA (6). The management of PE encompassed medical therapy only, thoracentesis, and/or surgical drainage with or without decortication (2-11). Most of the authors treated early and late PE with thoracentesis or chest drainage (2,3,6,8,10,11), while VATS with pleurodesis was reserved only for patients with PE that persisted after repeating thoracentesis and/or chest drainage (5,7,9). All studies but one (8) do not include follow-up, making difficult to define the effectiveness of thoracentesis or chest drainage to resolve PE, as it remains unknown in how many patients an effusion recurs. No case of recurrence was found after a VATS procedure; VATS was a safe procedure with a morbidity of 17% and no mortality (5,7,9). In patients for whom there was a significant delay before proceeding to VATS, it was not unusual to discover a trapped lung (5,7,9) that required in three cases a thoracotomy decortication (2,5,7). Most of papers were published before 2002 (2-6), thus the lack of familiarity with VATS could explain its limited and delayed use. VATS has become a routine procedure with a very low morbidity. Often the procedure can be performed through a single incision no bigger than that needed for chest tube placement. Thus, in patients with recurrent effusions after repeated thoracentesis, VATS allowed efficient deloculation of the pleura, proper positioning of chest drainage, and a thorough covering of the visceral and parietal pleura surface with smaller and safer talc dosage compared to that achieved with a talc slurry infused blindly through a chest drainage. This strategy could be useful in patients undergoing CABG using LITA as graft. Many surgeons open an entire length of pleura during harvesting LITA as a pedicle fashion, and do not close the pleura. As the result, a part of LITA goes through the pleural cavity. When the blind distribution of talc slurry is performed, IMA graft can be exposed to it and subsequent inflammation can ruin LITA graft. In theory, performing talc slurry under VATS guidance could prevent this possible complication. In three cases reported by Charniot et al. (9), the LITA graft was associated with the opening of the left pleural cavity and two of these patients had an off-pump beating-heart CABG. After VATS pleurodesis, in all cases no damage of LITA patency was observed. Similarly, Paull et al. (7) found a normal LITA patency after VATS pleurodesis. The limit of VATS compared to simple thoracentesis and/or chest drainage could be the increasing cost of the procedure since the VATS needs an operative room with a dedicated anaesthetic staff. However, as above reported in patients who underwent delayed VATS, it was common to identify the formation of tenacious peel that trapped the lung and required a conversion to thoracotomy to decorticate the inflammatory peel that covered the pleura and did not allow the lung re-expansion. Thus, the possibility of preventing future and more invasive procedure could turn in favour of choosing VATS as first strategy rather than less expensive procedures as chest drainage in case of PE unresponsive to thoracentesis. However, this strategy is supported by only five papers (2,5,7,9) with a low grade of evidence. The main limits of this analysis are (I) the lack of RCTs; (II) the retrospective nature of all studies; and (III) the lack of studies comparing VATS versus other conservative measurements. Thus, future RCTs are wanted to define the real effectiveness of VATS pleurodesis in the management of PE after CABG.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Heidecker J, Sahn SA. The spectrum of pleural effusions after coronary artery bypass grafting surgery. Clin Chest Med 2006;27:267-83. [Crossref] [PubMed]
- Kollef MH, Peller T, Knodel A, et al. Delayed pleuropulmonary complications following coronary artery revascularization with the internal mammary artery. Chest 1988;94:68-71. [Crossref] [PubMed]
- Hurlbut D, Myers ML, Lefcoe M, et al. Pleuropulmonary morbidity: internal thoracic artery versus saphenous vein graft. Ann Thorac Surg 1990;50:959-64. [Crossref] [PubMed]
- Sadikot RT, Rogers JT, Cheng DS, et al. Pleural fluid characteristics of patients with symptomatic pleural effusion after coronary artery bypass graft surgery. Arch Intern Med 2000;160:2665-8. [Crossref] [PubMed]
- Lee YC, Vaz MA, Ely KA, et al. Symptomatic persistent post-coronary artery bypass graft pleural effusions requiring operative treatment: clinical and histologic features. Chest 2001;119:795-800. [Crossref] [PubMed]
- Light RW, Rogers JT, Moyers JP, et al. Prevalence and clinical course of pleural effusions at 30 days after coronary artery and cardiac surgery. Am J Respir Crit Care Med 2002;166:1567-71. [Crossref] [PubMed]
- Paull DE, Delahanty TJ, Weber FJ, et al. Thoracoscopic talc pleurodesis for recurrent, symptomatic pleural effusion following cardiac operations. Surg Laparosc Endosc Percutan Tech 2003;13:339-44. [Crossref] [PubMed]
- Peng MC, Hou CJ, Li JY, et al. Prevalence of symptomatic large pleural effusions first diagnosed more than 30 days after coronary artery bypass graft surgery. Respirology 2007;12:122-6. [Crossref] [PubMed]
- Charniot JC, Zerhouni K, Kambouchner M, et al. Persistent symptomatic pleural effusion following coronary bypass surgery: clinical and histologic features, and treatment. Heart Vessels 2007;22:16-20. [Crossref] [PubMed]
- Labidi M, Baillot R, Dionne B, et al. Pleural effusions following cardiac surgery: prevalence, risk factors, and clinical features. Chest 2009;136:1604-11. [Crossref] [PubMed]
- El Nahal N, Abdel-Aal M, Nageb A, et al. Incidence and Management of Pleural Effusion after Coronary Artery Bypass Grafting Surgery. J Egypt Soc Cardiothorac Surg 2009;17:147-50.
- Centre for Evidence Based Medicine. Available online: http://www.cebm.net


