|
Original Article
Post-study therapy as a source of confounding in survival analysis of first-line studies in patients with advanced non-small-cell lung cancer
1Institute for Stroke and Dementia Research, Ludwig-Maximilians-Universität München, Munich, Germany; 2Institut für Medizinische Statistik und Epidemiologie, Klinikum rechts der Isar, Technische Universität München, Germany; 3Oncology Department, Asklepios Lungenfachkliniken München-Gauting, Germany
|
|
Abstract
Clinical trials exploring the long-term effects of first-line therapy in patients with advanced non-small-cell lung cancer generally disregard subsequent treatment although most patients receive second and third-line therapies. The choice of further therapy depends on critical intermediate events such as disease progression and it is usually left at the physician’s discretion. Time-dependent confounding may then arise with standard survival analyses producing biased effect estimates, even in randomized trials. Herein we describe the concept of time-dependent confounding in detail and discuss whether the response to first-line treatment may be a potential time-dependent confounding factor for survival in the context of subsequent therapy. A prospective observational study of 406 patients with advanced non-small-cell lung cancer served as an example base. There is evidence that time-dependent confounding may occur in multivariate survival analysis after first-line therapy when disregarding subsequent treatment. In the light of this important but underestimated aspect some of the large and meaningful recent clinical first-line lung cancer studies are discussed, focussing on subsequent treatment and its potential impact on the survival of the study patients. No recently performed lung cancer trial applied adequate statistical analyses despite the frequent use of subsequent therapies. In conclusion, effect estimates from standard survival analysis may be biased even in randomized controlled trials because of time-dependent confounding. To adequately assess treatment effects on long-term outcomes appropriate statistical analyses need to take subsequent treatment into account.
Key words
non-small-cell lung cancer; first-line therapy; survival analysis; post-study therapy; time-dependent confounding
J Thorac Dis 2011;3:88-98. DOI: 10.3978/j.issn.2072-1439.2010.12.07
|
|
Introduction
Advanced non-small-cell lung cancer (NSCLC) is the leading cause of cancer-related death (1). Currently there is no universally accepted standard regimen for the first-line treatment of advanced NSCLC. Still platinum-based combination chemotherapy is recommended as first choice. Here the question whether carboplatin is as effective as cisplatin is controversially discussed (2). With the availability of second- and third-line anti-cancer agents such as docetaxel, pemetrexed and erlotinib, and a greater acceptance for more aggressive therapy the majority of patients receive therapy beyond first-line (3). Especially many participants of clinical first-line trials as good risk patients are offered additional therapy.
In this paper we describe the concept of time-dependent confounding which may contribute to bias in the outcome measures of oncology trials. Therefore, we used the patient cohort from the oncology department of the Asklepios Lungenfachkliniken Muenchen-Gauting to detect whether response to first-line therapy may be a potential confounding factor in survival analysis. The most recent large and pivotal first-line NSCLC studies published from 2008 to 2010 were reviewed for the strategies used by the authors to account for post-study therapy and the way they discussed the resulting potential impact on the observed results.
|
|
The problem of endpoints in oncology trials
In view of the growing number of possible drugs, combinations, sequences, and settings to be tested for various diseases, the choice of endpoints in oncology trials is becoming a critical issue. There is increasing controversy about valid outcome
measures in oncology trials, especially in the first-line setting.
Overall survival (OS) is accepted as the most reliable and
relevant endpoint. Its drawback is that - depending on the natural
course of the disease – it may take a long time until the expected
event is observed. Furthermore it is subject to all therapeutic
measures applied in the course of an individual patient’s disease.
Thus, patient OS may well be influenced by the use of post-study
therapy (4). As a consequence, Itaya et al (5) proposed to use
the surrogate end point progression-free survival (PFS) as the
primary outcome measure in first-line trials in order to overcome
potential confounding by subsequent treatment. But reliable
evidence of relevant clinical benefits or advantages is not given
by using PFS, as extensively reviewed recently (6). A weakness
rather than strength of PFS compared to OS is that it does not
reveal insight into the real long-term impact and/or benefit of
the investigational treatment (6).
|
|
The concept of time-dependent confounding
The estimation of unbiased effect estimates should definitively
be the goal in clinical trials. However, standard methods for
survival analysis, such as time-dependent Cox proportional
hazards model, may produce biased effect estimates, regardless
of whether one further adjusts for covariate history. In this
context there is a potential bias caused by “time-dependent
confounding”, and this concept may apply, whenever: (a) there
exists a time-dependent covariate for mortality that also predicts
subsequent treatment and (b) this covariate is not independent
of previous treatment history (7-10).
Condition (a) implies that the measured covariate (for
example response to or performance status after therapy) may be
a confounder for the following treatment that must be adjusted
for. On the other hand condition (b) implies that the covariate
may also be affected by the previous treatment and thus, being
an intermediate variable (i.e. a step between treatment and
mortality), it should not be adjusted for by standard methods (9).
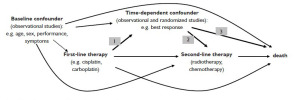
This complex problem is illustrated for the example of a
general cancer therapy study by the directed acyclic graph in
Figure 1. Superiority studies investigating the effectiveness of
first-line therapies hypothesize a significant difference in the
outcome (for example response) between the treatment arms
(arrow 1 in Figure 1). As indicated by meta-analyses (11,12),
there is strong evidence for a higher efficacy of cisplatin over
carboplatin with regard to tumor response. Therefore condition
(b) was met for these drugs in a hypothetical study context.
Response to first-line therapy has been shown to be an
independent predictor of mortality (as stated by 13-16 and
shown as arrow 3 in Figure 1). If response additionally predicts,
i.e. influences the choice of subsequent treatment (arrow 2 in
Figure 1) then condition (a) is also met. It is to be assumed that the choice of second-line treatment will differ in dependence of
the quality of the response achieved to the previous treatment
regimen. However, information about this association is scarce.
The decision on how therapy is being continued after firstline
treatment is usually made on an individual basis, and it is
thought to be influenced by the kind of drug used at first-line,
the response to first-line treatment, adverse reactions, early
discontinuation of first-line treatment, actual performance status
and other individual patient characteristics (17-20). In a recent
study (20), physicians were requested to assess the primary
reason for selecting a specific chemotherapy by completing
multiple choice forms. Main motivation was perception of
efficacy in this study.
|
|
The potential consequences of time-dependent
confounding for clinical trials
What are the possible consequences of these considerations? If,
for example, an ineffective first-line treatment in a given patient
may guide the decision on an effective second-line regimen,
then this decision may lead to a longer OS which is then falsely
attributed to the in fact less effective first-line regimen.
At least in large studies randomization prevents confounding
by a comparable distribution of baseline characteristics
and therefore prognostic factors between treatment groups
(Figure 1, dashed arrows from baseline confounder to first-line
treatment, 21). However, the choice of further therapy after
disease progression is usually at the physician’s discretion, also
in randomized trials. Time-dependent confounding may then
become a problem, especially in first-line studies because of the
high probability of subsequent lines of therapy.
In our own patient cohort about 30% of the patients actively
treated were clinical trial participants within different firstline
studies for advanced NSCLC (3,16). More than 60% of
these participants in clinical first-line trials received a secondline
therapy, about 35% a third-line therapy, and about 20%
radiotherapy after first-line systemic therapy. An association
between first-line treatment (platinum-based compared to not
platinum-based therapy) and disease control (DC, defined as any
response and disease stabilization) and between DC and OS has
already been shown and is published for this study population
(16). To fulfill the criteria of a potential time-dependent
confounder DC in addition would have to be associated with
subsequent treatment. In order to reveal such an association we
exemplarily investigated if the use of subsequent treatment is
indeed associated with the quality of response achieved to firstline
treatment in an own cohort of patients.
|
|
Analysis of an own cohort of patients with
advanced NSCLC regarding the association
between response to first-line treatment and subsequent therapy
Patients and methods
Patients with histologically confirmed NSCLC with stage IIIB
wet or stage IV were included in this prospective exploratory
observational study. Between January 2003 and July 2007, 519
patients with untreated advanced NSCLC were admitted to
a single ward of the Asklepios Lungenfachkliniken Gauting.
Of these patients, 406 were treated at our center systemically.
Patients were followed-up until August 2010. For further
description of the study population and data collection see
Zietemann and Duell (3,16).
Tumor evaluation
Tumor evaluation was performed according to our internal
standards. CT scans of the chest covering the upper abdomen
including liver and adrenal glands and of the brain were carried
out every 6 weeks during therapy and every 12 weeks in therapyfree
intervals unless indicated by the worsening or development
of clinical symptoms. Tumor response was evaluated semiquantitatively
(categories: partial response, stable disease,
progressive disease), a practice routinely applied in everyday
clinical practice. Disease progression was defined as an
appearance of new lesions or a clinically relevant growth or
deterioration of known lesions and/or symptoms.
Statistical analyses
To investigate time-dependent confounding with respect to
further-line treatments, we have chosen the variable DC after
first-line treatment as a possible time-dependent confounder
because DC rate was found to be a good predictor of OS in our
and in other studies (13,15,16).
To assess the association between response and the initiation
of second-line treatment (radiotherapy and chemotherapy, arrow
2 in Figure 1), the χ2-test was applied (22). Baseline variables
(sex, age, stage of disease, histology, Karnofsky performance
score, weight loss as symptom, smoking habit, and metastasis
location) were included as adjustment covariates and factors
within multiple logistic regression analyses considering initiation
of radiotherapy or chemotherapy as dependent variable (23).
Cox proportional hazard models were used to consider the
time between end of first-line and initiation of second-line
chemotherapy in the model 24. Odds ratios (OR) and hazard
ratios (HR) were reported with 95% confidence intervals. To
adjust for the continuous confounder “age” we used the SAS
macro %RCS_Reg (restricted cubic spline functions) in all
multiple analyses 25. All analyses were two-sided, conducted
at a 0.05 level of significance and carried out using SAS version
9.1 (SAS Institute Inc, Cary, NC).
Results – Analyses of time-dependent confounding
Association between response to first-line and the use of systemic
second-line therapy
Initiation of second-line therapy was less frequent in patients not
achieving DC (Table 1; with DC: 60%, without DC: 47%; χ2-
test: p-value=0.014). However, this difference was not significant
after adjustment for covariates (ORadjusted=0.91; 95%CI: 0.54-
1.52).
Median time between stop of first-line and start of second-line
was 117 days for patients with DC (inter quartile range (IQR):
71 to 188) and 23 days for patients without DC (IQR: 16 to 48).
Achieving no DC was associated with a higher probability of an early initiation of second-line chemotherapy (HRadjusted=4.07;
95%CI: 2.71-6.13).
Association between response to first-line therapy and subsequent
radiotherapy
Initiation of radiotherapy differed significantly depending on
best response after first-line treatment (Table 1; χ2-test: p-value
|
|
Evaluation of our results
The analyses from our prospective observational study support
the hypothesis that time-dependent confounding may bias the
results of standard survival analyses in the first-line treatment
of NSCLC. We found relevant associations between first-line
treatment and response (16), between response and survival
(16), and, as shown here, between response and the initiation of
therapy after first-line systemic therapy.
Our observational study has limitations, but also strengths:
because there was no patient selection for inclusion, the
results represent the treatment situation in every-day clinical
practice. Since data derive from only one department of a single
institution the results cannot be generalized as self selection
cannot be ruled out. Because confounding is a main problem in
observational studies unmeasured confounding may have biased
our results as well. Not using exact tumor measurement, such as
RECIST, reflects our every-day clinical practice where we focus
mainly on clinical criteria of response and clinical benefit rather
than on tumor shrinkage. Because we had detailed information
about radio- as well as systemic therapy after first-line treatment
we could analyse the impact of response to initiation of both,
including a large amount of possible confounding factors.
|
|
Review on recently published clinical first-line trials
regarding post-study therapy in the light of timedependent
confounding
As shown above, biased effect estimates may be obtained also in
randomized first-line trials, whenever the choice of subsequent
therapy may be influenced by the outcome of the previous line
of treatment. Although some studies provided information on
subsequent treatment separately for the treatment groups (for
example for cisplatin- compared to carboplatin-based firstline
therapy (26-30)), the potential impact of further-line
treatments on survival has rarely substantially been considered.
Fossella et al (26) reported that second-line treatment did not
confound survival results in favour of therapy with docetaxel
plus platinum, but they did not explain how they came to this
conclusion. Belani et al (27) mentioned possible confounding of survival as a result of a different use of taxanes second-line.
To our knowledge studies comparing cisplatin and carboplatin
at first-line adjusting for different subsequent treatments are not
available and therefore bias may have influenced the results of
studies comparing these two drugs.
In 2000, docetaxel was the first cytotoxic agent to be
registered for second-line treatment, pemetrexed at the end
of 2004, and erlotinib followed soon after. Because of the
increasing treatment options for second- (and even further-)
line and the growing number of patients receiving more than
one line of therapy it becomes more and more important to
take the influence of subsequent lines of therapy on survival
into consideration (31,32). Especially first-line trials initiated
after 2004 are therefore potentially subject to time-dependent
confounding. In this light we reviewed recently performed or
published larger phase III first-line studies. We found some
recent studies who did not mention post-study therapy at all (33-
35), including one study investigating the effect of early versus
late second-line docetaxel (36).
Table 2 summarizes seven large studies with inclusion period
starting 2004 who at least gave some information about poststudy
treatment (1,37-42). Information about radiotherapy after
first-line treatment was given only by three studies (1,38,41).
Three studies did not discuss a possible impact on OS (table 1; 37-39) and one stated that there was no impact on the final
results because of similar proportions of patients in both arms
having received second-line therapy, although the groups differed
regarding the post-study use of gemcitabine and docetaxel (40).
regarding the post-study use of gemcitabine and docetaxel (40).
In the large cetuximab (FLEX) study it was stated that in the
experimental arm post-study use of tyrosine kinase inhibitors
was less frequent than in the chemotherapy-alone arm (38),
which indicates that in unblinded studies the drugs used in prior
lines influence the choice on those used later on.
One study which discussed the possible impact of post-study
medication more in detail is the recently published AVAiL study
(1). An exploratory OS analysis for patients who did not receive
post-protocol therapy was performed. However, restriction to
those patients without post-study-treatment may introduce bias
because adjusting for a confounded intermediate will induce
confounding even if the exposure is randomized (10,21,43).
The problem of time-dependent confounding is not only
limited to the first-line setting but also to “maintenance” studies
(44-46). In general it is an issue whenever “treatment by
indication” is given (47). The data needed to correctly adjust
for time-dependent confounding are notoriously difficult to
collect, and many studies collect information on the class of drug
administered upon progression, only.
Table 1. Subsequent therapies depending on response to first-line therapy (Patients may have received more than one drug; cells are not mutually exclusive). 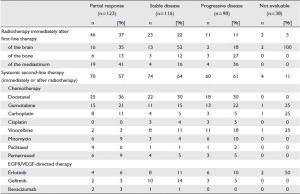
EGFR: epidermal-growth-factor-receptor; VEGF: vascular-endothelial-growth-factor Table 2. Characteristics of recently published large randomized phase III first-line trials in patients with advanced NSCLC 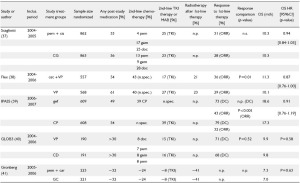
Table 2. Characteristics of recently published large randomized phase III first-line trials in patients with advanced NSCLC(continued) 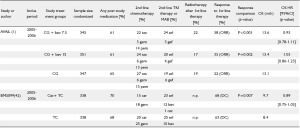
TKI: epidermal-growth-factor-receptor kinase inhibitors (erlotinib, gefitinib); MAB: monoclonal antibody (bevacizumab; cetuximab); chemotherapy: (cisplatin, carboplatin, docetaxel, paclitaxel, gemcitabine, vinorelbine, mitomycin, pemetrexed); DC: disease control rate; ORR: overall response rate; OS: overall survival; HR: hazard ratio; n.spec: not specified; n.p.: not presented; mth: months; bev: bevacizumab; car: carboplatin; cet: cetuximab; cis: cisplatin; doc: docetaxel; erl: erlotinib; gef: gefitinib; gem: gemcitabine; pem: pemetrexed; tax: taxane; VP: cisplatin + vinorelbine; CG: cisplatin + gemcitabine; CD: cisplatin + docetaxel; CP: carboplatin + paclitaxel; GC: carboplatin + gemcitabine; TC: carboplatin + paclitaxel or docetaxel Table 3. . Given information about poststudy therapy and discussion about the possible impact of poststudy therapy on OS 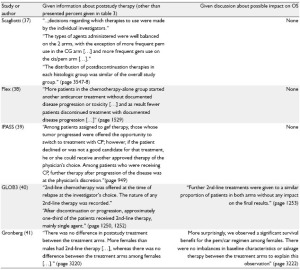
Table 3. . Given information about poststudy therapy and discussion about the possible impact of poststudy therapy on OS (continued) 
OS: overall survival; bev: bevacizumab; car: carboplatin; cet: cetuximab; cis: cisplatin; doc: docetaxel; erl: erlotinib; gef: gefitinib; gem: gemcitabine; pem: pemetrexed; tax: taxane; VP: cisplatin + vinorelbine; CG: cisplatin + gemcitabine; CD: cisplatin + docetaxel; CP: carboplatin + paclitaxel; GC: carboplatin + gemcitabine; TC: carboplatin + paclitaxel or docetaxel |
|
Discussion
The importance of respecting information about anticancer systemic therapy, radiotherapy and surgical intervention during
the post-study period is slowly entering the world of cancer trials.
But in which extent this information is used in the statistical
analysis is usually not revealed (45). In many published studies it
is discussed that the impact of post-study therapy on survival was
difficult to evaluate because the choice of subsequent treatment
is left to the discretion of the investigators (46). Statements
like “the selection of post-study treatment did not appear to
influence the overall survival conclusion” (46) or “the fact that
a small equal number of patients in each arm had second-line
treatment and no response was observed shows that secondline
treatment did not influence the survival data” (48) can be
found, but they are usually made without adequate scientific and
statistical evidence.
One way to overcome this problem was to predefine the
post-study treatment at study entry/randomization in order
to avoid bias introduced by such imbalances discussed above.
Alternatively, new statistical methods are available to estimate
the causal effect of time-dependent exposure in the presence of
time-dependent confounders, i.e. marginal structural models
and structural nested models (7,8,10). However, only one
study could be detected using causal models to adjust for
differential proportions of second-line treatment measures
(radiotherapy and chemotherapy) in cancer clinical trials
comparing cisplatin plus irinotecan with cisplatin plus vindesine
(49,50). Unfortunately sample size was small and the results
may therefore be unstable. Furthermore, exact information on
each patient’s treatment history was not presented and it was
assumed in the statistical model that the effect of second-line
radiotherapy was maintained up to time of death once it was
initiated (50). We could show, alike Yamagucchi and Ohashi
(50), that the decision of the physician for the initiation of
radiotherapy after first-line chemotherapy is associated with the
response to first-line treatment. We furthermore identified sex,
histology and brain metastases at baseline as relevant factors for
initiation of radiotherapy, and Karnofsky performance score and
bone metastases at baseline as relevant factors for initiation of
chemotherapy (data not shown).
|
|
Conclusion and future perspectives
Conclusion and future perspectives between response and outcome,
but we want to emphasize the
consequences resulting from
these associations.
At present our own ongoing
study is underpowered for
complex analyses like marginal
structural models and structural
nested models. Our future aim
is to analyse the data using
standard methods and causal
models and to investigate the
impact of time-dependent
confounding. Results obtained
from our own analysis and the
literature indicate that response
may be one of many potential
time-dependent confounders
in survival analysis following
first-line therapy. The treatment
flow of patients with advanced
cancer is being determined
by a complex combination of
dynamic and static influence
factors. Effect estimates may
be biased especially if dynamic
variables are not explicitly
accounted for in the analyses
(10). Future trials should
take subsequent treatments
and adjustment for t imed
ependent confounding
into consideration. Detailed
documentation of subsequent
treatment as well as other
covariates influencing the
respective treatment decision
process is a must in future
studies, otherwise even the
best statistical approaches
will fail to reveal the complex
interdependence between
the patients individual
characteristics, the biology of
the disease, and the therapeutic
measures applied. To draw
conclusions about optimal
treatment strategies further
analyses including all relevant
time-independent and timedependent
confounding factors are necessary.
|
|
Acknowledgments
We thank Prof. Loems Ziegler-Heitbrock for critical comments
on the manuscript.
|
|
References
Cite this article as: Zietemann VD, Schuster T, Duell THG. Post-study therapy as a source of confounding in survival analysis of first-line studies in patients with advanced non-small-cell lung cancer. J Thorac Dis 2011;3(2):88-98. doi: 10.3978/j.issn.2072-1439.2010.12.07
|