The impact of the 3-year ABSORB II trial results on my clinical practice: an Italian survey
Introduction
Since the introduction of bioresorbable vascular scaffold (BVS) as an alternative to the last generation drug eluting stent, randomized clinical trials attempted to evaluate efficacy and safety of these devices. Preliminary studies and data from registries suggested the safety of BVS, but these studies were limited to mid-term follow-up (1-5). The recent presentation and publication of the 3-year results of the “A clinical evaluation to compare the safety, efficacy and performance of ABSORB everolimus eluting bioresorbable vascular scaffold system against XIENCE everolimus eluting coronary stent system in the treatment of subjects with ischemic heart disease caused by de novo native coronary artery lesions” (ABSORB II) trial tried to fill this gap (6). In accordance with data from the first registries, the ABSORB II results reinforced concerns about major risk of device-oriented composite outcome failing to show superiority on vasomotion restoration and non-inferiority in late-lumen loss at 3 years. Despite the publication of these data incited the interest of the cardiologic community, the applicability of these results in everyday clinical practice is still controversial. This is particularly related to endpoints selection and implantation technique of the scaffold. In order to investigate how Italian interventional cardiologists implemented ABSORB II results in their everyday clinical practice, we decided to conduct a survey among the major interventional cardiology centers across Italy.
Methods
In January 2017, after the presentation and publication of the 3-year result of the ABSORB II trial, we prepared a simple questionnaire. The questionnaire was composed of 19 questions (18 multiple choice questions and 1 essay question). The complete list of questions is available in the supplemental materials. Briefly, the questions were selected to obtain the following main details: volume of the centre, experience of the operator, prior experience with BVS, knowledge of ABSORB II trial (design, endpoints, results), implication of the ABSORB II results in the daily clinical practice, potential explanations of study results, technique of BVS implantation, future directions of BVS. Starting from the list of Italian cath-labs (available in the website of the Società Italiana di Cardiologia Interventistica, SICI-GISE, https://gise.it), we selected the centers routinely implanting BVS. We contacted at least one operator for cath-lab (95 operators from 80 cath-labs overall). The expert was invited via e-mail and when we could not obtain a response, two reminders were sent after 5 and 10 days. The effectiveness of this approach has been tested and validated in a previous survey (7). STATISTICA 8 (Statsoft Inc., Tulsa, Okla, USA) was used to elaborate data.
Results
The detailed list of questions and replies is reported in the supplemental online. Below, we reported a brief summary of our survey’s results. Overall, 65 interventional cardiologists from 60 cath-labs completed the survey. We obtained a good representation of the country, receiving replies from 15 out of 20 Italian regions (Emilia Romagna, Lombardia, Veneto, Sicilia, Piemonte, Campania, Toscana, Sardegna, Marche, Liguria, Puglia, Friuli-Venezia Giulia, Valle D’Aosta, Lazio and Calabria).
Main characteristics of the operators involved in the survey
The majority of the operators (68%) work in high volume centers that perform more than 750 percutaneous coronary intervention (PCI)/year. Similarly, the majority of operators perform more than 200 PCI/year as first operator and 89% have been implanting scaffolds from at least 3 years. It is important to note that 37% of the operators implanted more than 100 scaffolds, while 35% implanted between 50 and 100 scaffolds. All the operators were aware about the publication of the study and the 1-year results, while only 2 were unaware about the publication of the 3-year results.
ABSORB II results: opinion of the operators and influence in the daily clinical practice
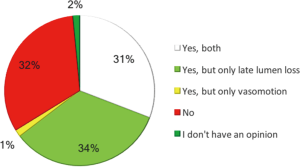
The opinion of the operators regarding the two main endpoints of the ABSORB II study was conflicting. We observed an interesting disagreement between operators. Thirty-one percent of the operators considered the two co-primary endpoints (late lumen loss and vasomotion) both reliable and reflecting clinical practice; 34% considered only late lumen loss reliable and interesting, whereas 32% showed a negative impression and consideration of both endpoints (Figure 1).
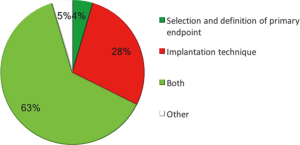
With regard to the impact on clinical practice, 60% of the operators declared that the results from the study did not change at all their patient selection and/or implantation technique and 72% declared that the number of scaffold implanted per year in their cath-lab will not change after the ABSORB II results. When asked to propose a potential explanation for the negative results, operators answered consistently (Figure 2), since 91% of them considered the implantation technique the main limit of the ABSORB II study and the major determinant of the negative results. Twenty-eight percent of the operators considered meaningful only the implantation technique, whereas 63% deemed important both implantation technique and definition of primary endpoints.
Implantation technique and daily clinical practice
The majority of the operators showed a well-established knowledge of the PSP technique (pre-dilatation, sizing to 1:1, post-dilatation with noncompliant balloon) (8-11). Sixty-one percent of the operators affirmed that they use the PSP technique in all the cases of BSV implantation, while 29% use it in more than 75% of cases. Accordingly, the majority of the operators will not change their practice, whereas in the remaining portion of the operators (23%), the implementation of the PSP technique will increase significantly. Of note, 77% of operators considered equally important all the aspects of the implantation technique, 9% considered sizing and postdilatation as most relevant, 8% only predilatation and 5% only postdilatation. The large majority (91%) of the operators was sure that a systematic application of the PSP technique could have influenced positively the results of the study.
Future perspectives on BVS
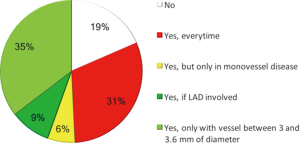
Thirty-seven percent of the respondents expect a use of scaffold devices in their cath-lab major than 10% at 5 years, while 45% between 5% and 10%. As for the ideal patient for BVS, 31% considered patients younger than 75 years and admitted for ST-segment elevation myocardial infarction as good candidates for scaffold implantation, while 35% of them preferred patients with target vessel diameter between 3 and 3.6 mm, and 9% of them preferred patients in whom left anterior descending artery is involved (12). A minority of the operators (18%) did not consider scaffold implantation a possible choice in patients admitted to hospital for ST-segment elevation myocardial infarction (Figure 3).
Discussion
The ABSORB II trial was a prospective, randomised, active-controlled, single-blind, parallel two-group, multicentre clinical trial enrolling patients with evidence of myocardial ischaemia and one or two de-novo native lesions in different epicardial vessels, assigned to receive treatment with an everolimus-eluting bioresorbable scaffold (ABSORB; Abbott Vascular, Santa Clara, CA, USA) or treatment with an everolimus-eluting metallic stent (XIENCE; Abbott Vascular, Santa Clara, CA, USA). The primary endpoint was superiority of the ABSORB bioresorbable scaffold versus the XIENCE metallic stent in angiographic vasomotor reactivity after administration of intracoronary nitrate. The co-primary endpoint was the non-inferiority of angiographic late luminal loss. The trial did not meet its co-primary endpoints and a higher rate of device-oriented composite endpoint due to target vessel myocardial infarction was observed in the Absorb group.
The aim of this survey was to assess the real applicability and the impact of these results in the everyday practice of interventional cardiologists.
Data analysis shows clearly how the population target of this survey works in high volume centers and consists of experienced operators which are well trained in scaffolds implantation. Additionally, they appear to be updated on scaffold literature, having a good knowledge of the ABSORB II study design and results. Focusing on the study design, there is widespread opinion that primary endpoint of vasomotion cannot find real applicability in routine clinical practice since there is no actual evidence regarding its impact on clinical outcome. Furthermore, it is evident how the implantation technique is considered by Italian interventional cardiologists a very meaningful aspect during scaffold positioning, ascribing the same importance to predilatation, sizing and postdilatation. Since the validation of the PSP technique was quite recent, this consideration supports the idea (firmly consolidated in our survey population) that lack of specific implantation expertise may have played a major role in the failure of the study. Furthermore, as Tamburino et al. described in a consensus paper (13), predilatation, correct sizing and postdilatation with non-compliant balloon should be always performed during scaffold implantation. This practice resulted well established between Italian interventional cardiologists.
Recently, Ortega-Paz et al. published an analysis from the GHOST-EU registry (14), trying to evaluate scaffold implantation by three different scoring model and finding significantly incidence reduction of device oriented composite outcome (DOCE) with fewer cases of definite/probable scaffold thrombosis in patients in which optimal scaffold implantation was performed compared to those in which there was suboptimal implantation process, highlighting again the importance of a rigorous and accurate approach to scaffold implantation.
It is worthy to note that, after the start of our survey, several storm clouds are gathering over BVS safety, with particular regard to early and late scaffold thrombosis. The “everolimus-eluting bioresorbable vascular scaffolds in patients with coronary artery disease” (ABSORB III) trial 2-year results showed a significantly higher rate of target lesion failure (TLF) at 2 years (11% vs. 7.9%, P=0.03) with a significantly higher rate of target vessel-myocardial infarction (TV-MI) (7.3% vs. 4.9%, P=0.04) in the BVS group (15). Moreover, “the Amsterdam investigator-initiated absorb strategy all-comers trial (AIDA)” trial deeply questioned BVS safety (16). The results were released due to the high rate of definite/probable scaffold thrombosis in the BVS group (3.5% at 2 years) with a worrisome number of late adverse events. As a consequence, Abbott Vascular decided to withdraw BVS from EU market in order to collect prospective data from experienced operators with standardized implantation technique. This data could make our survey look a little outdated. The amount of negative results on BVS may suggest that operators tend to overestimate the role of technical skill to determine the device-related outcome. From one side, it could be really interesting to repeat a similar survey on this new data in a broader, international context, in order to understand the feeling of international operators about BVS results. On the other side, we think that operators’ awareness regarding correct implantation is not an overestimation since even in the recently published data on BVS several appraisals on implantation technique could be made.
Conclusions
ABSORB II trial did not influence clinical practice among Italian interventional cardiologists mainly due to the overall idea that the co-primary endpoints were not adequate to provide a robust evidence on device clinical safety and also because the lack of experience on device implantation may have influenced the outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ormiston JA, Serruys PW, Onuma Y, et al. First serial assessment at 6 months and 2 years of the second generation of absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study. Circ Cardiovasc Interv 2012;5:620-32. [Crossref] [PubMed]
- Serruys PW, Onuma Y, Dudek D, et al. Evaluation of the second generation of a bioresorbable everolimus-eluting vascular scaffold for the treatment of de novo coronary artery stenosis: 12 month clinical and imaging outcomes. J Am Coll Cardiol 2011;58:1578-88. [Crossref] [PubMed]
- Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention 2015;10:1144-53. [Crossref] [PubMed]
- Jaguszewski M, Ghadri JR, Zipponi M, et al. Feasibility of second-generation bioresorbable vascular scaffold implantation in complex anatomical and clinical scenarios. Clin Res Cardiol 2015;104:124-35. [Crossref] [PubMed]
- Kraak RP, Hassell ME, Grundeken MJ, et al. Initial experience and clinical evaluation of the Absorb bioresorbable vascular scaffold (BVS) in real-world practice: the AMC Single Centre Real World PCI Registry. EuroIntervention 2015;10:1160-8. [Crossref] [PubMed]
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016;388:2479-91. [Crossref] [PubMed]
- Tebaldi M, Biscaglia S, Pecoraro A, et al. Fractional flow reserve implementation in daily clinical practice: A European survey. Int J Cardiol 2016;207:206-7. [Crossref] [PubMed]
- Biscaglia S, Ugo F, Ielasi A, et al. Bioresorbable Scaffold vs. Second Generation Drug Eluting Stent in Long Coronary Lesions requiring Overlap: A Propensity-Matched Comparison (the UNDERDOGS study). Int J Cardiol 2016;208:40-5. [Crossref] [PubMed]
- Biscaglia S, Campo G, Tebaldi M, et al. Bioresorbable vascular scaffold overlap evaluation with optical coherence tomography after implantation with or without enhanced stent visualization system (WOLFIE study): a two-centre prospective comparison. Int J Cardiovasc Imaging 2016;32:211-23. [Crossref] [PubMed]
- Biscaglia S, Secco G, Tumscitz C, et al. Optical coherence tomography evaluation of overlapping everolimus-eluting bioresorbable vascular scaffold implantation guided by enhanced stent visualization system. Int J Cardiol 2015;182:1-3. [Crossref] [PubMed]
- Imori Y, D'Ascenzo F, Gori T, et al. Impact of postdilatation on performance of bioresorbable vascular scaffolds in patients with acute coronary syndrome compared with everolimus-eluting stents: A propensity score-matched analysis from a multicenter "real-world" registry. Cardiol J 2016;23:374-83. [Crossref] [PubMed]
- Ielasi A, Varricchio A, Campo G, et al. A prospective evaluation of a standardized strategy for the use of a polymeric everolimus-eluting bioresorbable scaffold in ST-segment elevation myocardial infarction: Rationale and design of the BVS STEMI STRATEGY-IT study. Catheter Cardiovasc Interv 2017;89:1129-38. [Crossref] [PubMed]
- Tamburino C, Latib A, van Geuns RJ, et al. Contemporary practice and technical aspects in coronary intervention with bioresorbable scaffolds: a European perspective. EuroIntervention 2015;11:45-52. [Crossref] [PubMed]
- Ortega-Paz L, Capodanno D, Gori T, et al. Predilation, sizing and post-dilation scoring in patients undergoing everolimus-eluting bioresorbable scaffold implantation for prediction of cardiac adverse events: development and internal validation of the PSP score. EuroIntervention 2017;12:2110-7. [Crossref] [PubMed]
- Ellis SG, Kereiakes DJ, Stone GW, et al. Everolimus-eluting Bioresorbable Vascular Scaffolds in Patients with Coronary Artery Disease: ABSORB III Trial 2-Year Results. Presented by Dr. Stephen Ellis at the American College of Cardiology Annual Scientific Session (ACC 2017). Washington, DC, March 18, 2017.
- Wykrzykowska JJ, Kraak RP, Hofma SH, et al. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N Engl J Med 2017;376:2319-28. [Crossref] [PubMed]


