Heart-resident macrophages: are they involved in the rhythm of every beat?
Macrophages are one of the main components of the innate immune system and play a major role in cardiovascular disease (1,2). These cells are derived from blood monocytes and differentiate into tissue macrophages. They are well known for their immunologic roles including phagocytosis and antigen presentation. The role of macrophages in normal and disease physiology, however, is much more complex as illustrated by the fact that they can develop into distinct functional phenotypes depending upon their microenvironment. Different sub-populations of macrophages have been characterized, of which M1 “inflammatory” macrophages and “pro-healing” M2 macrophages are the most studied (3).
Alongside blood monocyte-derived macrophages, specialized tissue resident cells bearing macrophages markers carry out functions distinct to their location. These include microglia in the brain, brown adipose tissue macrophages, Kupffer cells in the liver, alveolar macrophages in the lung, and osteoclasts in the bone. Resident macrophages also exist within the heart and are known to participate in a variety of pathophysiological processes such as post-myocardial infarction healing (4,5). Although monocyte-derived macrophages and resident tissue macrophages are commonly addressed as similar types of cells, they have different embryonal origins, turnover rates, and fates (6). Our understanding of the roles of these tissue-resident cells is still evolving.
One of the essential functions of the heart is as a pacemaker which regulates its own beat, essential for the coordinating its pumping function. The cardiac conduction system is composed of a specialized group of cardiomyocytes which are responsible for initiating and transmitting electrical activity. The sinoatrial node located in the right atrium is the master pacemaker of the heart which sends electrical signals to the atrioventricular node (AVN) which coordinates contraction between the upper (atria) and lower (ventricles) chambers. Disorders of heart rhythm conduction are not uncommon and disruption of the AVN can lead to a variety of disorders, some of which are treated by inserting an artificial pacemaker via pacing wires into the right side of the heart. Until recently, it has been assumed that AVN conduction system of the heart is controlled exclusively by specialized cardiomyocytes within this node.
In a recent issue of the journal Cell, a study by Hulsmans et al. changes our understanding of the how the AVN conduction system works. The authors studied the relationship between cardiac resident cell with positive macrophage markers, and the specialized cardiac conduction system in the atrioventricular (AV) node (7). They show in both human and mice specimens that the specialized conduction tissue in the AV node contains more macrophages than the left ventricle (LV). These macrophages are spindle shaped with processes found in proximity to conduction system cardiomyocytes. Immunostaining for the gap junction protein-connexin 43, known for its ability to synchronize the electrical depolarization of adjacent cardiomyocytes, demonstrated its interposition between the cardiomyocytes and the resident cells bearing the macrophages markers. By using patch clamp technique, it was shown that these cells have positive effect on the excitability and a shortening of the refractory period of the AVN conduction tissue. Blocking connexin 43 blocked this effect. Using optogenetics directed to the monocyte lineage, Hulsmans et al. were able to modulate the conduction properties of the AV node by switching a light on and off during an electrophysiological study.
Origin of cardiac macrophages
Myocardial tissue-resident macrophages are primarily established prenatally and arise from embryonic yolk-sac progenitors. In situ proliferation allows them to perpetuate independently of circulating blood derived monocytes (8-10). In this paper, the authors performed parabiosis experiment of wild-type and CX3CR1-GFP mice, which express green fluorescent protein in monocytes as well as other immune cells, and demonstrated minimal (~1%) contribution of circulating cells to AV node resident macrophages. In aggregate, these experiments demonstrate a novel role for cardiac resident macrophages in electrical activation of the heart.
Possible role of cardiac macrophages in human disease
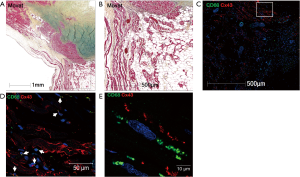
What then are the implications of this study for our understanding of the development and maintenance of normal cardiac conduction as well as for instances when conduction goes awry? These results raise the intriguing possibility that the conduction system macrophages described by Hulsmans et al. may play an important role not only in the maintenance of normal conduction but also how it responds to injury. Both the SA and AVN are subject to age-dependent fibrosis and disorders of cardiac conduction are not uncommon in elderly individuals. Could macrophages play a role in such age-dependent processes? In addition, infiltrating cardiac diseases, such as sarcoidosis and amyloidosis, change the ventricular and conduction system architecture. This is believed to be responsible for their arrhythmogenic effect, by producing re-entry circuits. In the figure (Figure 1), there is a pathohistological specimen of a young man, who died from sudden cardiac arrest, from our sudden cardiac arrest registry. Movat pentachrome staining demonstrated a fragmentation of the compact AV node, a finding that might suggest a fatal conduction defect or a re-entry arrhythmia as a cause of death. In immunofluorescence, one can observe the proximity of macrophages (stained with CD68 antibody in green) and cardiomyocytes with connexin 43 (stained red) in-between. Could the macrophages be contributing to the pathophysiology?
It is possible that cardiac macrophages in the setting of infection or inflammation might further modulate the refractory period and resting potential of cardiomyocytes resulting in predisposition towards arrhythmia? Inflammatory conditions such as Lyme carditis may also be modulated by these cells (11).
Future treatment targets
Elucidating a novel mechanism for cardiac conduction opens a new window of possibilities for therapeutic intervention. First, in inflammatory conditions in which conduction disturbances occur, selective targeting the culprit inflammatory mechanism might be beneficial for preventing or shortening the need for temporary pacing. Further, could cardiac macrophages be used for modulation of rhythm in other situations? A negative chronotropic effect can be achieved by removing the tonic effect of cardiac conduction system macrophages. Could their selective manipulation be a long-term or even permanent alternative to the use of beta-blockers, non-dihydropyridine Ca2+ channel blockers and ivabradine in patients who cannot tolerate these drugs? Targeting can be achieved by local administration of recombinant monoclonal anti-bodies to these cells using markers such as CSF-1r, CD68 or scavenger receptors. On the other hand, in conditions in which the conduction system has been damaged such as in an inferior myocardial infarction or more rarely in lev’s disease, facilitation of conduction might remove the need for external pacing. Another potential use would be to reduce the risk of fatal arrhythmias in patients with congenital of acquired long QT syndrome. As conduction system macrophages shorten the cardiomyocyte refractory period, it is reasonable to assume that augmenting this effect can shorten the QT interval both by its direct effect and by its positive chronotropic event. Like all great works in science, the paper by Hulsmans et al. raises more questions than it answers but opens the potential for a new era in our understanding of the electrophysiology of the heart.
Acknowledgements
Supported by CVPath Institute Inc.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 2013;13:709-21. [Crossref] [PubMed]
- van Dijk RA, Rijs K, Wezel A, et al. Systematic Evaluation of the Cellular Innate Immune Response During the Process of Human Atherosclerosis. J Am Heart Assoc 2016.5. [PubMed]
- Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol 2014;5:514. [Crossref] [PubMed]
- Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol 2015;35:1066-70. [Crossref] [PubMed]
- Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007;204:3037-47. [Crossref] [PubMed]
- Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol 2015;33:643-75. [Crossref] [PubMed]
- Hulsmans M, Clauss S, Xiao L, et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017;169:510-22.e20. [Crossref] [PubMed]
- Davies LC, Jenkins SJ, Allen JE, et al. Tissue-resident macrophages. Nat Immunol 2013;14:986-95. [Crossref] [PubMed]
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity 2014;41:21-35. [Crossref] [PubMed]
- Jenkins SJ, Ruckerl D, Cook PC, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 2011;332:1284-8. [Crossref] [PubMed]
- Lelovas P, Dontas I, Bassiakou E, et al. Cardiac implications of Lyme disease, diagnosis and therapeutic approach. Int J Cardiol 2008;129:15-21. [Crossref] [PubMed]


