Effects of preemptive cerebrospinal fluid drainage on spinal cord protection during thoracic endovascular aortic repair
Introduction
Thoracic endovascular aortic repair (TEVAR) has revolutionized the treatment of pathologies of the thoracic aorta, including thoracic aortic aneurysms, penetrating atherosclerotic ulcers, traumatic thoracic aortic injuries, and acute complicated type B aortic dissections, with reduced perioperative mortality and morbidity, in comparison with open repair (1). Although the incidence of spinal cord injury (SCI) after TEVAR is lower than that after open repair, it still ranges from 3% to 12% (2) and can lead to profound long-term disability. Various strategies have been suggested to reduce the risk of SCI, including cerebrospinal fluid drainage (CSFD) and maintaining an increased mean arterial pressure (MAP) to improve spinal cord perfusion. Protective protocols for SCI are generally used with open repair of thoraco-abdominal aortic aneurysms (TAAA), but these protocols are less defined for TEVAR (2). Current guidelines recommend the use of prophylactic CSFD with TEVAR for long-segment descending thoracic aortic coverage in patients with prior abdominal aortic aneurysm (AAA) repair (3). However, these guidelines are based on evidence from open repair of thoracic aortic pathologies, not from TEVAR (4,5). In addition, systematic reviews have failed to show that CSFD prevents SCI in TEVAR (5). Consequently, the protocols for SCI prevention in TEVAR vary widely, with some centers advocating the routine use of CSFD and others advocating the use of selective preoperative or postoperative CSFD (6).
The purpose of this study was to evaluate the overall incidence of SCI and determine the efficacy of prophylactic CSFD in preventing SCI after TEVAR.
Methods
Our institutional review board approved this study and waived the need for informed consent owing to its retrospective nature (Yonsei IRB No. 3-2014-0144).
Patients and data source
From January 2012 to August 2014, 162 patients underwent TEVAR for various aortic pathologies at Gangnam Severance Hospital, Yonsei University College of Medicine in Seoul, Korea. Among these patients, 81 underwent preoperative CSFD, whose data we retrospectively reviewed.
Preoperative, intraoperative, and postoperative variables were extracted from the computerized database of the hospital, with additional information obtained through a retrospective record review. Preoperative data included patient demographics [age, sex, and body mass index (BMI)], comorbidities (diabetes, hypertension, previous cerebrovascular accident, coronary artery occlusive disease, chronic obstructive pulmonary disease, peripheral artery occlusive disease, old cerebrovascular accident, and carotid artery disease), previous operations of the abdominal aorta, a dominant vertebral artery on computed tomography (CT), serum creatinine level (mg/dL), hemoglobin level (g/dL), and the time of CSFD insertion. Intraoperative data included the level of the landing zone, left subclavian artery (LSA) coverage, LSA revascularization, the number of stent grafts used, the type of stent grafts used, MAP at deployment, and final endoleak. Postoperative data included the incidence of paraplegia, CSFD complications, the distal level of the stent graft, and the length of aortic coverage on postoperative CT. The distal level of the stent graft was measured by using the spinal level, and the stent graft length of aortic coverage was measured manually on postoperative CT. Operative mortality was defined as in-hospital mortality or mortality within 30 days post operation. Survival data were obtained from a hospital chart review.
Clinical practice
All the operations were performed under general anesthesia in a hybrid operating room (OR). The need for perioperative adjuncts (e.g., CSFD, LSA revascularization, and percutaneous or open vessel access) was at the discretion of the attending surgeon and the interventional radiologist. Systemic heparinization (100 U/kg) was applied in all the patients to achieve an appropriate activated clotting time of >300 seconds. Oversized devices with diameters 10% to 15% greater than that of the aortic diameter of the proximal portion of the pathologic aorta were used.
CSFD indication and SCI prevention strategy
CSFDs were performed in high-risk patients for SCI during TEVAR. High-risk patients were defined as those who (I) received coverage of the LSA; (II) received extensive coverage of long segments of the thoracic aorta (length of the coverage by stent graft, >30 cm); (III) received prior downstream aorta repair; (IV) had compromising important intercostal (T8-L1), vertebral, pelvic and hypogastric collaterals; or (V) had a shaggy aorta (6). We performed CSFD prior to TEVAR in the patients with a high risk of SCI, including emergent cases. CSF was drained intermittently to achieve CSF pressures of <10 mmHg in the OR and intensive care unit (ICU). If no specific neurological problems occurred, the CSFD catheter was removed 48 hours after the procedures. However, if delayed neurological deficit occurred, CSF must be drained to achieve an intracranial pressure of <5 mmHg and to maintain a MAP of >90 mmHg (7).
Definition of SCI
SCI was defined as any new lower extremity sensory and/or motor deficit not attributable to epidural hematoma, peripheral neuropathy, or intracranial pathology. Postoperative neurological examination was performed in the OR or ICU whenever possible. Patients who had any new neurological deficit at the first postoperative neurological examination were classified as having an immediate SCI. Delayed SCI was defined as a newly developed SCI after the first postoperative neurological examination (8). All CSFDs were inserted by the neurosurgery team at our hospital. At the beginning of the study period, the CSFD was inserted the day before the operation in the general ward, but this was changed to take place in the OR after the induction of general anesthesia for the patients’ convenience and prevention of puncture site infection. LSA revascularization was routinely performed when the LSA was covered, except for emergent cases. All the patients were admitted to the ICU postoperatively.
Statistical analyses
Continuous variables were summarized by using mean values and standard deviations. The categorical variables were summarized by using frequencies and percentages. Independent risk factors of SCI were identified using univariate and multivariate logistic regression. All statistical analyses were performed using the Statistical Package for Social Sciences version 23 (SPSS, Chicago, Ill, USA).
Results
Demographics
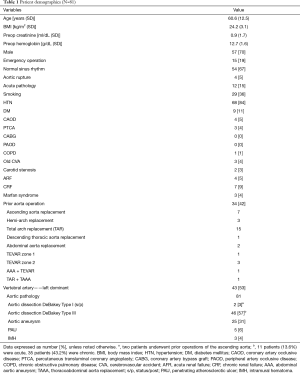
Patient demographics and comorbidities, as well as the types of aortic pathology, are shown in Table 1. The mean patient age was 60.6±12.5 years. Of the patients, 57 (70%) were male and 24 (30%) were female. Fifteen patients underwent emergency operations. Four patients (4.9%) had previously undergone abdominal aorta replacement.
Full table
Procedural details
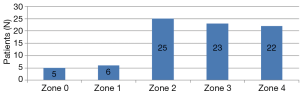
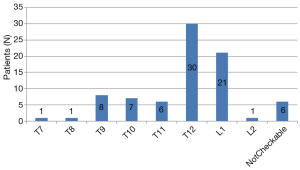
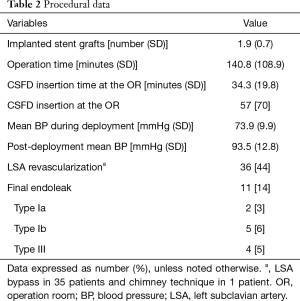
In 81 patients, 150 stent grafts were deployed, including the Zenith TX2 Proform (Cook, Bloomington, Ind, USA) in 46 (57%) patients, the Valiant Captivia (Medtronic, Minneapolis, Minn, USA) in 33 (41%), and both the TX2 and Valiant in 2 (3%). Twenty-five patients (31%) needed 1 stent graft; 45 (56%), 2 grafts; 9 (11%), 3 grafts; and 2 (3%), 4 grafts. One of the two patients, who required 4 stent grafts, underwent zone 0 TEVAR, and the total treatment length was 28.87 cm. The other patient requiring 4 stent grafts underwent zone 3 TEVAR with abdominal aorta to the superior mesenteric artery bypass, and the total treatment length was 32.61 cm. These two patients required four stent grafts, not only due to the long coverage length that was needed, but more so due to the difference in diameter between the proximal landing zone and the distal landing zone due to chronic aortic dissection. The mean stent graft length for aortic coverage was 22.4±6.2 cm. The proximal landing zones are shown in Figure 1. The level of the distal landing zone was checked by using postoperative CT (Figure 2). Eleven patients had endoleaks on their final angiogram, including 2 patients with type Ia endoleak, 5 with type Ib endoleak, and 4 patients with type III endoleak. None of the patients required conversion to open surgery. The endoleak recovered spontaneously in all the patients. Twenty-four patients (30%) underwent CSFD in the general ward the day before TEVAR, while 57 patients (70%) underwent CSFD at the OR after general anesthesia, with a mean time of CSFD insertion at the OR of 34.3±19.8 minutes. Of 36 patients (44%) who needed LSA coverage and underwent LSA revascularization, 35 underwent LSA bypass and the remaining patient underwent the chimney technique. The procedural data are shown in Table 2.
Full table
Perioperative outcomes
The perioperative data are shown in Table 3. The mean durations of hospital and ICU stay were 13.3 and 2.2 days, respectively. The mean duration of indwelling CSFD was 3 days. Four patients had postoperative cerebrovascular accidents, two of whom required rehabilitation because of unilateral weakness. All the 4 patients had antiplatelet medication. Although 23 patients (28%) experienced CSFD-related complications, most had minor complications (spinal headache in 11, puncture-site pain in 5, puncture-site infection in 1, radiating leg pain in 1, and intracranial hypotension in 3), except 2 patients with subdural hematoma. Of the two cases, one seemed to be related to the use of heparin right after catheter insertion. The other was due to intracranial hypotension. All the patients with CSFD-related complications recovered after proper conservative management, except for 1 patient who had to undergo an operation due to subdural hematoma. SCI occurred in 2 patients (2.5%). Of those patients, one recovered to ambulatory status at discharge after hypertensive therapy and the other had a permanent disability. No complications related to LSA revascularization occurred. Two patients (2.5%) died within 30 days after TEVAR, but neither had SCI. One of the patients died because of multiorgan failure, and the other patient died of an unknown cause after discharge to home. The mean follow-up durations of the patients who developed and those who did not develop SCI after TEVAR were 4.42±0.21 and 9.70±7.83 months, respectively (P=0.467).
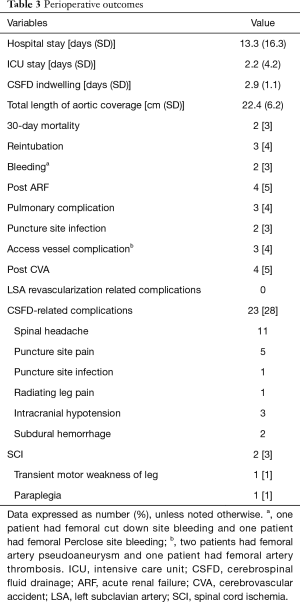
Full table
Risk factor analysis for SCI
Among the 81 patients with CSFD, 2 (2.5%) developed SCI after TEVAR. We analyzed the risk factors of SCI. Univariate logistic regression analysis revealed that sex, age, BMI, emergency operations, previous aortic operations, postoperative ARF, postoperative CVA, postoperative pulmonary complications, the level of the distal landing zone, and the length of aortic coverage were not significant prognostic factors of SCI. Previous AAA repair tended to be related to SCI (P=0.065). Multivariate logistic regression analysis revealed that preoperative aortic rupture was a significant independent risk factor of SCI (P=0.002; Table 4).
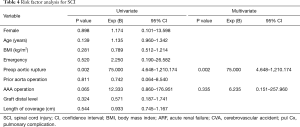
Full table
Discussion
SCI is a critical complication of open thoracic aortic surgery and TEVAR. SCI has been reported in up to 20% of open thoracic aortic surgeries (5). Various strategies to reduce SCI have been devised, including CSFD, cooling, intercostal vessel reimplantation, and increased MAP in open aortic surgery. A relationship between CSF pressure and the development of SCI during repairs of the thoracic aorta have been suggested in many studies. The rationale for CSFD is that spinal cord perfusion pressure is the difference between MAP and the CSF pressure (9). The use of prophylactic CSFD during open aortic surgery is controversial, and many studies have been conducted on this subject. Coselli et al. demonstrated a significant decrease in SCI when using CSFD in a large randomized trial (13% vs. 2.6%, P=0.03) (10). The benefit of prophylactic CSFD in open aortic surgery has been established by 2 meta-analyses (9,11). In TEVAR, the risk of SCI has not been completely established, with reported incidence rates ranging from 3% to 12% (2,12), and spinal cord-protective protocols are less defined than open aortic surgeries. Many studies have reported the incidence of SCI after TEVAR with CSFD, but no randomized trials have examined this issue. In the literature, risk factors that have been associated with SCI after TEVAR include emergency surgery, treatment of a long portion of the aortic segment, dissection, rupture, advanced age, and prior abdominal aortic operation or stent graft (7). The exclusion of intercostal arteries (T7-L1) supplying the anterior spinal artery is associated with neurological events, and long coverage of the thoracic aorta is a significant risk factor of SCI (13). With the increasing application of TEVAR for various thoracic aortic pathologies, the use of CSFD is becoming more important for minimizing neurological deficits. However, no consensus has been reached among vascular surgeons about the best strategy for CSFD.
Several studies support the use of CSFD as an adjunct, with few of these studies demonstrating associated complications (14-19). The overall complication rate of CSFD has been reported to be approximately 5%. The most severe complication was intracranial hemorrhage, which has been associated with a mortality rate as high as 50% (18,20).
During our earlier experience with TEVAR, we used CSFD more selectively in patients treated for a long portion of the aortic segment. Subsequently, we routinely used CSFD in our high-risk TEVAR patients, including emergent cases. In our series of 81 patients, 23 patients (28.4%) had CSFD-related complications, but most recovered quickly, except one patient who required surgery for subdural hemorrhage (SDH). This patient recovered well after surgery. Our study included minor complications such as mild headaches and pains, so the overall incidence of CSFD-related complications was high. Two patients (2.5%) who received CSFD developed SCI. One of the patients had transient motor weakness of the leg, while the other had paraplegia (Table 3). Among the patients who did not receive preoperative CSFD, one developed SCI. Wong et al. reported in a systematic review that the overall incidence of SCI after TEVAR was 3.88% [95% confidence interval (CI), 2.95–4.85]. The overall rate of SCI in our study was 1.85% in the patients with or without CSFD. Wong et al. indicated that the use of prophylactic CSFD was difficult to define on the basis of the existing literature. Prophylactic CSFDs were only used in patients at high risk of perioperative SCI, so the analysis had an inherent bias (5). Arnaoutakis et al. reported that their strategy using CSFD as an adjunctive procedure for TEVAR resulted in lower rates of SCI than those reported in prior studies that used selective CSFD, as well as increased unadjusted survival. Thus, they recommended preoperative CSFD for all eligible patients whenever clinically feasible (21). With the use of routine CSFD in this study, the incidence rate of permanent SCI in the high-risk group was 1.2%. This rate was lower than that reported in prior studies. Thus, prophylactic CSFD is a safe and effective therapy for reducing the risk of SCI, in spite of the related complications. The overall rate of CSFD-related complications, including minor complications, was 28%. Greater caution is needed to reduce the incidence of complications.
The LSA is the main artery to the left upper limb. LSA occlusion may cause various complications, including varying degrees of upper limb ischemia, cerebrovascular accident, and SCI (22). The LSA has 3 major branches associated with TEVAR, namely the left internal mammary artery, vertebral artery, and costocervical trunk. The vertebral artery and costocervical trunk contribute to spinal cord perfusion. Thus, the LSA coverage during TEVAR may induce SCI by reducing spinal blood flow (23). Spinal cord perfusion depends on more than one source of blood supply (22). Buth et al. demonstrated the clinical significance of this source of collateral perfusion of the spinal cord. They reported that the coverage of the LSA without revascularization was significantly associated with perioperative paraplegia or paraparesis in the EUROSTAR registry. In their study, neurological complications developed in 8.4% of the patients without LSA revascularization but in none of the patients with prophylactic LSA revascularization (P=0.049) (24). Cooper et al. demonstrated that the risk of SCI was increased in patients who required LSA coverage in a meta-analysis and systematic review (22). Several review studies support routine preemptive LSA revascularization (22,23,25,26). The Society for Vascular Surgery Practice guidelines recommend routine preoperative revascularization in patients who need LSA coverage during TEVAR, despite the very-low-quality evidence (grade 2, level C) (27). Peterson et al. demonstrated that LSA revascularization could be performed with a relatively low risk (28). In our study, no complications associated with LSA revascularization occurred. With the routine use of CSFD and LSA revascularization in this study, the incidence rate of SCI was lower than that reported in prior studies.
The mechanism underlying SCI is not completely understood but may relate to reperfusion injury and hemodynamic derangements. Many studies reported that an increased length of aortic coverage during TEVAR was associated with SCI (2,29-31). Keith et al. reported that longer stent graft coverage of the thoracic aorta was associated with an increased incidence of SCI, but no specified length was defined as a factor of increased risk of SCI (2). We demonstrated that preoperative aortic rupture was a risk factor of SCI. In patients with thoracic aortic rupture, more-aggressive strategies have been considered for preventing SCI, such as maintaining the MAP between 80 and 100 mmHg right after stent-graft deployment (7). In the literature, previous AAA operation and the length of the stent graft are known risk factors of SCI after TEVAR, but these were not associated with SCI in our study. This may be due to the small sample size used in our study.
Despite the perioperative advantages of TEVAR over open aortic repair, SCI remains a devastating complication that has a profound influence on long-term outcomes. In our data, patients who developed and those who did not develop SCI after TEVAR had mean lengths of postoperative survival of 4.42±0.21 and 9.70±7.83 months, respectively (P=0.467).
Limitations
Our study has some limitations. Its retrospective nature limited the variables available for analysis; therefore, some selection bias or unidentified confounding bias may have influenced the results. The patients in the study population were heterogeneous (including both emergency and elective patients with various aortic pathologies). In addition to the relatively small sample size, the selection of patients from a single center may have limited the generalizability of our results. We did not assess all possible causes of SCI and did not analyze the complete hemodynamic data following discharge.
Conclusions
Our preemptive CSFD as an adjunctive procedure to TEVAR proved to have better outcomes than the selective use of CSFD in other prior reports of SCI cases. Preoperative CSFD is recommended as a prophylactic procedure in patients at high risk of SCI during TEVAR.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our institutional review board approved this study and waived the need for informed consent owing to its retrospective nature (Yonsei IRB No. 3-2014-0144).
References
- Bavaria JE, Appoo JJ, Makaroun MS, et al. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg 2007;133:369-77. [Crossref] [PubMed]
- Keith CJ Jr, Passman MA, Carignan MJ, et al. Protocol implementation of selective postoperative lumbar spinal drainage after thoracic aortic endograft. J Vasc Surg 2012;55:1-8; discussion 8. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv 2010;76:E43-86. [Crossref] [PubMed]
- Khan SN, Stansby G. Cerebrospinal fluid drainage for thoracic and thoracoabdominal aortic aneurysm surgery. Cochrane Database Syst Rev 2012;10:CD003635. [PubMed]
- Wong CS, Healy D, Canning C, et al. A systematic review of spinal cord injury and cerebrospinal fluid drainage after thoracic aortic endografting. J Vasc Surg 2012;56:1438-47. [Crossref] [PubMed]
- Uchida N. How to prevent spinal cord injury during endovascular repair of thoracic aortic disease. Gen Thorac Cardiovasc Surg 2014;62:391-7. [Crossref] [PubMed]
- Lam CH, Vatakencherry G. Spinal cord protection with a cerebrospinal fluid drain in a patient undergoing thoracic endovascular aortic repair. J Vasc Interv Radiol 2010;21:1343-6. [Crossref] [PubMed]
- DeSart K, Scali ST, Feezor RJ, et al. Fate of patients with spinal cord ischemia complicating thoracic endovascular aortic repair. J Vasc Surg 2013;58:635-42.e2. [Crossref] [PubMed]
- Cinà CS, Abouzahr L, Arena GO, et al. Cerebrospinal fluid drainage to prevent paraplegia during thoracic and thoracoabdominal aortic aneurysm surgery: a systematic review and meta-analysis. J Vasc Surg 2004;40:36-44. [Crossref] [PubMed]
- Coselli JS, LeMaire SA, Koksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 2002;35:631-9. [Crossref] [PubMed]
- Khan SN, Stansby G. Cerebrospinal fluid drainage for thoracic and thoracoabdominal aortic aneurysm surgery. Cochrane Database Syst Rev 2004.CD003635. [PubMed]
- Drinkwater SL, Goebells A, Haydar A, et al. The incidence of spinal cord ischaemia following thoracic and thoracoabdominal aortic endovascular intervention. Eur J Vasc Endovasc Surg 2010;40:729-35. [Crossref] [PubMed]
- Preventza O, Wheatley GH 3rd, Williams J, et al. Identifying paraplegia risk associated with thoracic endografting. Asian Cardiovasc Thorac Ann 2009;17:568-72. [Crossref] [PubMed]
- McHardy FE, Bayly PJ, Wyatt MG. Fatal subdural haemorrhage following lumbar spinal drainage during repair of thoraco-abdominal aneurysm. Anaesthesia 2001;56:168-70. [Crossref] [PubMed]
- Cohen S, Shorshtein A, de La Calzada M, et al. Subarachnoid-cutaneous fistula and subdural hematoma complicating cerebrospinal fluid drainage for thoracoabdominal aorta aneurysm repair. J Clin Anesth 2006;18:475-6. [Crossref] [PubMed]
- Murakami H, Yoshida K, Hino Y, et al. Complications of cerebrospinal fluid drainage in thoracoabdominal aortic aneurysm repair. J Vasc Surg 2004;39:243-5. [Crossref] [PubMed]
- Weaver KD, Wiseman DB, Farber M, et al. Complications of lumbar drainage after thoracoabdominal aortic aneurysm repair. J Vasc Surg 2001;34:623-7. [Crossref] [PubMed]
- Dardik A, Perler BA, Roseborough GS, et al. Subdural hematoma after thoracoabdominal aortic aneurysm repair: an underreported complication of spinal fluid drainage? J Vasc Surg 2002;36:47-50. [Crossref] [PubMed]
- Estrera AL, Sheinbaum R, Miller CC, et al. Cerebrospinal fluid drainage during thoracic aortic repair: safety and current management. Ann Thorac Surg 2009;88:9-15; discussion 15. [Crossref] [PubMed]
- Wynn MM, Mell MW, Tefera G, et al. Complications of spinal fluid drainage in thoracoabdominal aortic aneurysm repair: a report of 486 patients treated from 1987 to 2008. J Vasc Surg 2009;49:29-34; discussion 34-5. [Crossref] [PubMed]
- Arnaoutakis DJ, Arnaoutakis GJ, Beaulieu RJ, et al. Results of adjunctive spinal drainage and/or left subclavian artery bypass in thoracic endovascular aortic repair. Ann Vasc Surg 2014;28:65-73. [Crossref] [PubMed]
- Cooper DG, Walsh SR, Sadat U, et al. Neurological complications after left subclavian artery coverage during thoracic endovascular aortic repair: a systematic review and meta-analysis. J Vasc Surg 2009;49:1594-601. [Crossref] [PubMed]
- Noor N, Sadat U, Hayes PD, et al. Management of the left subclavian artery during endovascular repair of the thoracic aorta. J Endovasc Ther 2008;15:168-76. [Crossref] [PubMed]
- Buth J, Harris PL, Hobo R, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: Incidence and risk factors. a study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg 2007;46:1103-10; discussion 10-1. [Crossref] [PubMed]
- Rizvi AZ, Murad MH, Fairman RM, et al. The effect of left subclavian artery coverage on morbidity and mortality in patients undergoing endovascular thoracic aortic interventions: a systematic review and meta-analysis. J Vasc Surg 2009;50:1159-69. [Crossref] [PubMed]
- Weigang E, Parker JA, Czerny M, et al. Should intentional endovascular stent-graft coverage of the left subclavian artery be preceded by prophylactic revascularisation? Eur J Cardiothorac Surg 2011;40:858-68. [PubMed]
- Matsumura JS, Lee WA, Mitchell RS, et al. The Society for Vascular Surgery Practice Guidelines: management of the left subclavian artery with thoracic endovascular aortic repair. J Vasc Surg 2009;50:1155-8. [Crossref] [PubMed]
- Peterson BG, Eskandari MK, Gleason TG, et al. Utility of left subclavian artery revascularization in association with endoluminal repair of acute and chronic thoracic aortic pathology. J Vasc Surg 2006;43:433-9. [Crossref] [PubMed]
- Feezor RJ, Martin TD, Hess PJ Jr, et al. Extent of aortic coverage and incidence of spinal cord ischemia after thoracic endovascular aneurysm repair. Ann Thorac Surg 2008;86:1809-14; discussion 1814.
- Matsuda H, Ogino H, Fukuda T, et al. Multidisciplinary approach to prevent spinal cord ischemia after thoracic endovascular aneurysm repair for distal descending aorta. Ann Thorac Surg 2010;90:561-5. [Crossref] [PubMed]
- Kotelis D, Geisbusch P, Hinz U, et al. Short and midterm results after left subclavian artery coverage during endovascular repair of the thoracic aorta. J Vasc Surg 2009;50:1285-92. [Crossref] [PubMed]