Chylothorax after pediatric cardiac surgery complicates short-term but not long-term outcomes—a propensity matched analysis
Introduction
Postoperative chylothorax in pediatric patients undergoing congenital heart surgery is a rare and serious complication that causes prolonged stays in intensive care units (ICUs). In these cases, the most important precipitating factors are increased pressure in the systemic venous circulation, surgical injury of the thoracic duct during operations or thrombus formation in a central vein (1-3).
Accumulation of the lipid and protein rich chyle in the pleural space leads to malnutrition and prolonged mechanical ventilation and poses an increased risk of infection. Treatment can be classified into two categories: conservative and surgical. The conservative approach consists of replacing the nutrients lost with the chyle and using chest drainage if necessary (4). Studies have demonstrated the effectiveness of a low fatty acid and medium-chain triglyceride (MCT) diet (3,5), which can be followed by total parenteral nutrition (TPN) (4,6,7) and octreotide therapy if needed (8-10). Surgical therapies, such as supraphrenic ligation of the thoracic duct (11-14) or parietal pleurectomy and pleurodesis (15) can lead to recovery if conservative treatment is ineffective.
The aim of our study was to identify the perioperative characteristics of chylothorax patients using the 10-year database of a tertiary national pediatric cardiac center. We analyzed the treatment modalities and their success rate in our patients. Additionally, we assessed the long term outcomes of these patients.
Methods
A total of 1,664 consecutive pediatric patients undergoing heart surgery were admitted to our cardiac ICU between January 2004 and December 2008. During this period, 24 patients had and 1,640 did not have chylothorax. Therefore, we extended patient data collection for the occurrence of chylothorax for 10 years. The incidence of chylothorax has been reported as the percentage of an indexed type of surgery between 2002–2012. The study was approved by the Regional Ethical Committee and the Institutional Review Board (25980/2012/EKU). A propensity-matched statistical method allowed for analyses of two groups of patients with similar characteristics.
The diagnosis of chylothorax was made on biochemical testing and a positive chest X-ray: pleural spaces with high levels of triglycerides (>110 mg/dL), proteins (>20 g/L), and lymphocytes (>80% of cells) (3). Patients with solely transudate formation were excluded.
The cardiac surgical procedures were graded by applying risk adjustment for the congenital heart surgery (RACHS) scores (16). The vasoactive inotropic scores (VIS) were calculated as: dopamine dose (µg/kg/min) + dobutamine dose (µg/kg/min) + 100× epinephrine dose (µg/kg/min) + 10× milrinone dose (µg/kg/min) + 10,000× vasopressin dose (U/kg/min) + 100× norepinephrine dose (µg/kg/min) (17).
The in-hospital endpoints were mortality, serious morbidity and resource utilization. This latter includes the need for mechanical ventilation, the length of ICU and in-hospital stays and the need for renal replacement therapy. We defined mortality as in-hospital death from any reason, including children who died after having been transferred to another hospital. Postoperative low cardiac output syndrome [LCOS; clinical signs (tachycardia, hepatomegaly, cardiac arrest), with a base excess lower than −4 mmol/L or a lactate level higher than 2 mmol/L in two consecutive arterial blood samples, a UO lower than 1 mL/kg/hr, a maximum VIS higher than 20, or the need for mechanical circulatory support]; pulmonary failure (non-infectious and non-vascular oxygenation problems, such as atelectasis, pneumothorax, chylothorax, phrenic paresis); renal failure (need for peritoneal dialysis or hemodialysis) and infection (catheter-related and deep sternal wound infection, positive hemoculture or sepsis) were considered to be adverse outcomes. Neurological events, such as convulsion without previous anamnesis, hemorrhage or cerebral infarction demonstrated on cranial imaging, were also included as complications (17).
We assessed the long-term outcomes and complications. The follow-up period ended on November 15, 2016. During this follow-up period, the number and outcomes of the reoperations, morbidities, neurodevelopmental outcomes, confirmed thrombophilia and/or thromboembolic events were also recorded.
Statistical analysis
The results are expressed as counts and percentages as well as the mean and standard deviation (SD) for categorical and continuous variables, respectively. The demographic and perioperative differences between patients were compared basing on a χ2-test, Fisher’s exact test and t-tests, as appropriate.
Due to the differences in baseline characteristics, the group with chylothorax and control group were not comparable with respect to important covariates. We established a propensity score model for the two groups; thus, we minimized differences and reduced the bias resulting from the study design. The propensity score was constructed using a multivariable logistic regression model, with chylothorax as the binomial dependent variable and all of the measured covariates that could be related to chylothorax (14 variables) as predictor variables (18) and which were: age (days), RACHS points, preoperative ICU stay (days), preoperative need for inotropes, preoperative need for captopril, CPB time (minutes), aortic cross-clamp time (minutes), operation time (minutes), nasal temperature (degree Celsius), deep hypothermic cardiac arrest (DHCA), need for nitric oxide, fluid balance, need for RBC transfusion and need for aprotinin.
The variables had a P<0.2 value in the univariate analysis. The Hosmer-Lemeshow test and c-index were used to measure the model’s reliability and predictive ability, respectively. The receiver operating characteristic curve’s c-index (area under the curve) was 0.8345, and the Hosmer-Lemeshow C statistic was 11.3, with a P value of 0.183 (8 degrees of freedom).
Chylothorax patients were matched to patients without this complication with similar propensity scores. A 1:1 nearest-neighbor greedy matching without replacement to form pairs using calipers was applied. The width was equal to 0.2 of the SD of the logit of the propensity score (19). The 48 matched pairs were analyzed for differences in their baseline characteristics and outcome variables. We realized that the treatment options were different in patients with a vascular ring. Therefore, in the tables reporting postoperative and long-term complications, these six patients and their pairs were excluded. The standardized differences were estimated to evaluate the effectiveness of balancing the baseline characteristics between the two groups (20). The standardized differences were between −0.1 and 0.1 across the 14 baseline covariates (21). All of the tests were two-sided, and the endpoints and measured covariates were compared with a nonparametric-test for continuous variables and McNemar’s test for the categorical data. SPSS 21 statistical software (SPSS, Chicago, IL, USA) was used. A P value of less than 0.05 was considered statistically significant. The follow-up extended from the day of discharge to the date of death or censoring, and its median was computed using the Kaplan-Meyer method.
Results
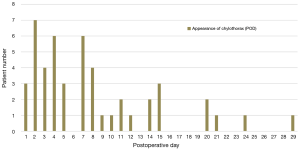
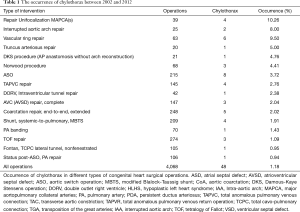
During the 10-year period, 48 patients had chylothorax after pediatric cardiac surgery, and its incidence was 1.1%. One patient had chylothorax preoperatively and one patient had additional significant anomalies in the area of the esophagus and the larynx. Chylothorax was observed between the first (3 patients, 6.2%) and 29th (1 patient, 2%) postoperative days. The highest incidence of chylothorax was observed on the second postoperative day (7 patients, 14.6%). Seven patients (14.6% of the chylothorax population) died during their in-hospital stay. Causes of death were, in 2 of the cases infection with consequent severe septic states; in 2 other cases, untreatable low-output syndrome; in 1 case fatal respiratory failure due to a residual pulmonary vein obstruction, which could not have been resolved by a surgical procedure; in 1 other case fatal thrombotic complications, followed by a superior vena cava syndrome and in 1 case irreversible pulmonary hypertension with acute respiratory failure after pleurodesis. In the control group mortality rate for the same period was 4.8% (80 patients of 1,640). Among the chylothorax patients, eleven had genetic abnormalities (3 Down’s syndrome, 3 Di-Giorge’s syndrome, 1 IgA deficiency and 1 VACTERL, 3 minor anomalies). The distribution of the congenital heart operations and individual occurrence of chylothorax during the 10-year period is shown in Figure 1 and Table 1. High postoperative venous pressures (central venous pressure above 18 mmHg) were observed in 23 patients (48%). We had two cases (4.2%) in which the central venous cannulation was the possible cause of ductal injury.
Full table
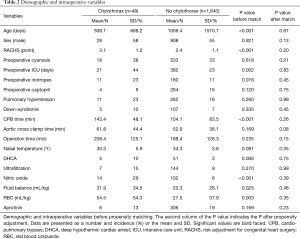
After excluding those who had chylothorax after a vascular ring operation (6 patients), the propensity score matching yielded 42 pairs of patients in our database. Before propensity matching, we found higher values of the perioperative variables in patients with chylothorax: these children were younger than the control patients (P<0.001), had higher RACHS points (P<0.001), and their CPB times were significantly longer (P<0.001). Patients with chylothorax required more blood products (P=0.003) and were treated with a greater volume in the first 72 hours (P=0.025). Chylothorax patients needed higher doses of inotropes and nitric-oxide intraoperatively (P=0.016 and P<0.001, respectively). The demographic and perioperative characteristics before matching are presented in Table 2. After propensity matching, we could not determine any significant difference in our statistically balanced system regarding the pre-and intraoperative variables.
Full table
There were no significant differences in the postoperative mortality and composite mortality. The occurrence of pulmonary failure (P=0.001) and severe infection (P=0.06) was higher in the chylothorax group. They also required prolonged mechanical ventilation (P=0.002), but the neurological events, LCOS and renal failure did not differ significantly between the groups. The lengths of the ICU and in-hospital stays (P=0.05 and P=0.01) were longer in the chylothorax group compared to the matched population (Table 2).
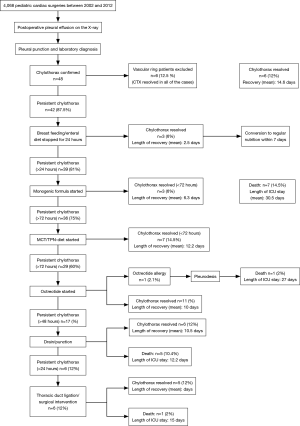
We used early surgical treatment in patients with vascular ring operations. In the majority of cases, after establishing the diagnosis, conservative therapy was started. For those patients who were refractory to the monogenic formula diet and cessation of breast feeding, MCT-TPN therapy was initiated. Twenty-nine patients who did not respond to TPN therapy received octreotide infusion. One patient had an octreotide allergy. Thirty-four patients (70.8%) had permanent thoracic drainage. Early surgical intervention (<24 hours) was needed in those 6 patients who had vascular ring operations, while in 7 cases, early surgical intervention was performed after unsuccessful medical therapy. The therapeutic algorithm is presented in Figure 2. During the long-term follow up, neither octreotide treatment nor surgical ligation were associated with increased mortality (log-rank P=0.54 and P=0.91, respectively).
The mean survival times were 11.2 years (95% CI: 9.9–13.4 years) and 10.6 years (95% CI: 8.7–12.5 years) in the chylothorax group and control group, respectively (log-rank test: 0. 47). During the long-term follow-up period, additional 3 patients have died in the chylothorax group [10 patients (23.8%) during the 10 years] and 12 patients (28.2%) in the control group. Causes of death in the chylothorax group were in 1 case pneumonia induced septic shock and in 2 cases a cerebrovascular event—1 intracranial hemorrhage with consequent herniation and 1 ischemic complication). In the chylothorax group, two-thirds of patients needed at least one reoperation within the first 2 years, which was slightly more than in the control group. During the follow-up, 4 patients had thromboembolic complications (2 had confirmed thrombophilia) in the chylothorax group, while three events were observed in the control group. Neurologic complications (hypoxic cerebral lesions diagnosed by MRI) and epilepsy were frequent in both groups. In the chylothorax group, phrenic palsy occurred more frequently (P=0.04). During the 49 reoperations, chylothorax did not reoccur. The results are shown in Table 3 and the follow-up data in Table 4.

Full table
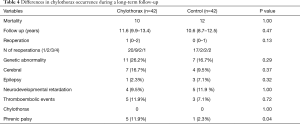
Full table
Discussion
We have found that the occurrence of chylothorax was 1.1% in our population and the highest incidence occurred on the second postoperative day. Using propensity score analysis, patients with chylothorax had more pulmonary complications and required prolonged mechanical ventilation. Their in-hospital stays were longer compared to the control. There was no difference in the complications regarding the treatment options. During the 49 operations of the 48 chylothorax patients, chylothorax did not reoccur. The mortality and morbidity rates were similar during the mean 11.2 years of survival.
In our study population, the incidence of chylothorax was 1.1% over a 10-year period, which was slightly lower than the 1.18% of occurrence reported in the literature (for a population of 4,068 patients) (2,8,22). However, it is possible that the incidence is not related to the annual surgical volume. Usually, chylothorax appears between day 0–10 after injury to the thoracic duct, except in patients undergoing a vascular ring operation in which the surgical injury is certain (22). In the present study, the peak appearance of chylothorax was observed on the second postoperative day, which raises the possibility that chylothorax is a result of multiple precipitating factors presenting in the postoperative period. These factors could be high systemic venous pressures, thrombotic states or high chylomicron loads by initiation of breast milk feeding (though nasogastric tube). Interestingly, chylothorax did not reoccur during the reoperations, which supports the hypothesis of a multifactorial origin.
The presence of major aortopulmonary collateral arteries (MAPCAs) or a single ventricle physiology and operations with high venous pressures and arch repairs are associated with an increased occurrence of chylothorax (3,22).
Chylothorax is not only an obscure complication, it is also associated with prolonged mechanical ventilation and an increased length of ICU and in-hospital stays. Higher rates of sepsis and an increased need for inotropic support were also reported. Sepsis was found to be associated with the initiation of TPN, continuous chest drainage and immunosuppression (23). Higher mortality rates associated with chylothorax were reported after adjusting for operation complexity, age and hospital volume (24). By contrast, the lack of significant differences in mortality rates was also reported between chylothorax groups and control patients (22). Our analysis used a propensity score adjustment, and based on our data, we showed that the lengths of mechanical ventilation and hospital stays were longer in the chylothorax patients compared to the matched cohort. Using the same propensity matched cohort for comparison, we did not observe higher rates in the mortality rate, neurologic events or thrombophilic complications, except for the slightly higher rates of phrenic palsy.
The effectiveness of treatment protocols for chylothorax was continuously examined. Among those children who underwent vascular ring operations, the first-line surgical intervention was 100% successful. In any other case, the initial management was the same: first, breastfeeding was stopped and then enteral nutrition with MCT nutrition and TPN (3,4,24-26), followed by the initiation of octreotide (27-33) therapy. The surgical interventions were thoracic drainage and a ligature of the thoracic duct (2,34). Studies reported that a MCT diet was effective in 71% of patients and that TPN as a first-line treatment should not be used. Among those patients who did not respond to the MCT-diet, thrombosis of the subclavian vein was revealed and thrombolysis was a successful treatment. TPN, in contrast to a MCT diet, has more side effects and is not more effective than other therapies (35). In our study, a MCT diet was only successful in 14.5% of cases (7 patients of 36). Certain centers advise that enteral feeding should be stopped and TPN should be used in each case if the patient has elevated venous pressures (>15 mmHg), and it is necessary to consider the risk of higher nosocomial infection rates and side-effects (2). The effectiveness of octreotide therapy is still unclear. Some studies have reported that in a few cases, the infusion could lead to the elimination of chylothorax; however, the factors causing inefficiency were also named. Fontan and Glenn operations, with postoperative higher venous pressures, were refractory to the somatostatin analogue therapy (26,33). We should highlight that after the diagnosis of chylothorax, a MCT diet was started, and if the patient required continuous chest drainage 24 hours later (i.e., re-accumulation of chyle in the chest), TPN was started; only after 4 days was octreotide therapy started. In accordance with other cardiac centers, we also suggest a step-by-step institutional guideline for early treatment to prevent further resource utilization.
After ineffective octreotide therapy, surgical treatment should be considered. Open thoracotomy, especially with mass ligation of the right thoracic duct, is suitable when both sides of the chest are involved (14). Duct ligation can reduce the need for chest tube drainage in the majority of patients, especially for those who previously had higher venous pressures associated with chylothorax since they are more vulnerable to earlier death and higher rates of mortality (36). Certain publications recommend surgery within 5–7 days to limit the morbidity and mortality associated with the procedures (22,37). The majority of chylothorax patients respond to conservative pharmacological therapy or thoracic duct ligation, and there is no need for more complicated interventions, such as pleurodesis, pleurectomy, direct wound ligation or en masse supradiaphragmatic ligation (22). We had one patient who had an octreotide allergy, and subsequently, pleurodesis was performed. According to our data, the long-term outcomes were not influenced by the type of treatment for chylothorax.
The present study had some limitations. The retrospective analysis did not allow us to find the relationship between causes and consequences. However, we were able to prospectively maintain a database on all of the pediatric ICU patients since 2002, and we therefore were able to perform long-term outcome research. Our study was single-centered and the patient population was heterogeneous regarding age and congenital heart defects, which could influence the generalizability of the results. We applied propensity matched analysis to balance the bias based on the heterogeneity of patients and operation characteristics.
Conclusions
Chylothorax is a rare complication after pediatric cardiac surgery. High venous pressures, certain operation procedures and congenital abnormalities were more often present in these cases, and the fact that chylothorax did not reoccur in these patients might indicate a multifactorial origin of this disease. Based on our results, the length of hospital stay and resource utilization were higher in the chylothorax group after propensity score adjustment. However, chylothorax was not associated with increased mortality or a higher risk for long-term complications.
Acknowledgements
The authors thank the employees of the Ambulance of Cardiology and the employees of the Pediatric Cardiac Intensive Care Unit of the Gottsegen György Hungarian Institute of Cardiology. We also thank Fekete, Roland Kodácsi and György Róth, medical and PhD students of the Semmelweis University for their help in collecting data and creating the database. This work received departmental funding only.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Regional Ethic Committee (TUKEB) approved the study (No. 25980/2012/EKU; 454/PI/12). The individual parental consent was waived by the Ethical Committee Board.
References
- Van Veldhuizen PJ, Taylor S. Chylothorax: a complication of a left subclavian vein thrombosis. Am J Clin Oncol 1996;19:99-101. [Crossref] [PubMed]
- Panthongviriyakul C, Bines JE. Post-operative chylothorax in children: an evidence-based management algorithm. J Paediatr Child Health 2008;44:716-21. [Crossref] [PubMed]
- Biewer ES, Zurn C, Arnold R, et al. Chylothorax after surgery on congenital heart disease in newborns and infants -risk factors and efficacy of MCT-diet. J Cardiothorac Surg 2010;5:127. [Crossref] [PubMed]
- McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med 2010;104:1-8. [Crossref] [PubMed]
- Bond SJ, Guzzetta PC, Snyder ML, et al. Management of pediatric postoperative chylothorax. Ann Thorac Surg 1993;56:469-72; discussion 72-3. [Crossref] [PubMed]
- Fernández Alvarez JR, Kalache KD, Grauel EL. Management of spontaneous congenital chylothorax: oral medium-chain triglycerides versus total parenteral nutrition. Am J Perinatol 1999;16:415-20. [Crossref] [PubMed]
- de Beer HG, Mol MJ, Janssen JP. Chylothorax. Neth J Med 2000;56:25-31. [Crossref] [PubMed]
- Pratap U, Slavik Z, Ofoe VD, et al. Octreotide to treat postoperative chylothorax after cardiac operations in children. Ann Thorac Surg 2001;72:1740-2. [Crossref] [PubMed]
- Rosti L, De Battisti F, Butera G, et al. Octreotide in the management of postoperative chylothorax. Pediatr Cardiol 2005;26:440-3. [Crossref] [PubMed]
- Roehr CC, Jung A, Proquitte H, et al. Somatostatin or octreotide as treatment options for chylothorax in young children: a systematic review. Intensive Care Med 2006;32:650-7. [Crossref] [PubMed]
- Graham DD, McGahren ED, Tribble CG, et al. Use of video-assisted thoracic surgery in the treatment of chylothorax. Ann Thorac Surg 1994;57:1507-11; discussion 11-2. [Crossref] [PubMed]
- Kent RB 3rd, Pinson TW. Thoracoscopic ligation of the thoracic duct. Surg Endosc 1993;7:52-3. [Crossref] [PubMed]
- Sieczka EM, Harvey JC. Early thoracic duct ligation for postoperative chylothorax. J Surg Oncol 1996;61:56-60. [Crossref] [PubMed]
- Liu CS, Tsai HL, Chin TW, et al. Surgical treatment of chylothorax caused by cardiothoracic surgery in children. J Chin Med Assoc 2005;68:234-6. [Crossref] [PubMed]
- Browse NL, Allen DR, Wilson NM. Management of chylothorax. Br J Surg 1997;84:1711-6. [Crossref] [PubMed]
- Jenkins KJ. Risk adjustment for congenital heart surgery: the RACHS-1 method. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2004;7:180-4. [Crossref] [PubMed]
- Davidson J, Tong S, Hancock H, et al. Prospective validation of the vasoactive-inotropic score and correlation to short-term outcomes in neonates and infants after cardiothoracic surgery. Intensive Care Med 2012;38:1184-90. [Crossref] [PubMed]
- Tóth R, Szanto P, Prodan Z, et al. Down syndrome and postoperative complications after paediatric cardiac surgery: a propensity-matched analysis. Interact Cardiovasc Thorac Surg 2013;17:691-7. [Crossref] [PubMed]
- Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J 2009;51:171-84. [Crossref] [PubMed]
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. [Crossref] [PubMed]
- Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf 2008;17:1202-17. [Crossref] [PubMed]
- Ismail SR, Kabbani MS, Najm HK, et al. Impact of chylothorax on the early post operative outcome after pediatric cardiovascular surgery. J Saudi Heart Assoc 2014;26:87-92. [Crossref] [PubMed]
- Franksson C, Magnusson G, Ringdén O, et al. Drainage of thoracic duct lymph in renal transplant patients. Transplantation 1976;21:133-40. [Crossref] [PubMed]
- Mery CM, Moffett BS, Khan MS, et al. Incidence and treatment of chylothorax after cardiac surgery in children: analysis of a large multi-institution database. J Thorac Cardiovasc Surg 2014;147:678-86 e1; discussion 85-6.
- Milonakis M, Chatzis AC, Giannopoulos NM, et al. Etiology and management of chylothorax following pediatric heart surgery. J Card Surg 2009;24:369-73. [Crossref] [PubMed]
- Matsuo S, Takahashi G, Konishi A, et al. Management of refractory chylothorax after pediatric cardiovascular surgery. Pediatr Cardiol 2013;34:1094-9. [Crossref] [PubMed]
- Rimensberger PC, Muller-Schenker B, Kalangos A, et al. Treatment of a persistent postoperative chylothorax with somatostatin. Ann Thorac Surg 1998;66:253-4. [Crossref] [PubMed]
- Zuluaga MT. Chylothorax after surgery for congenital heart disease. Curr Opin Pediatr 2012;24:291-4. [Crossref] [PubMed]
- Landis MW, Butler D, Lim FY, et al. Octreotide for chylous effusions in congenital diaphragmatic hernia. J Pediatr Surg 2013;48:2226-9. [Crossref] [PubMed]
- Gómez-Caro Andrés A, Marron Fernandez C, Moradiellos Diez FJ, et al. Octreotide for conservative management of postoperative chylothorax. Arch Bronconeumol 2004;40:473-5. [Crossref] [PubMed]
- Lim KA, Kim SH, Huh J, et al. Somatostatin for postoperative chylothorax after surgery for children with congenital heart disease. J Korean Med Sci 2005;20:947-51. [Crossref] [PubMed]
- Caverly L, Rausch CM, da Cruz E, et al. Octreotide treatment of chylothorax in pediatric patients following cardiothoracic surgery. Congenit Heart Dis 2010;5:573-8. [Crossref] [PubMed]
- Landvoigt MT, Mullett CJ. Octreotide efficacy in the treatment of chylothoraces following cardiac surgery in infants and children. Pediatr Crit Care Med 2006;7:245-8. [Crossref] [PubMed]
- Soto-Martinez M, Massie J. Chylothorax: diagnosis and management in children. Paediatr Respir Rev 2009;10:199-207. [Crossref] [PubMed]
- Bhargava SK. Breast feeding best for the babies. Yojana 1983;27:29-30. [PubMed]
- Nath DS, Savla J, Khemani RG, et al. Thoracic duct ligation for persistent chylothorax after pediatric cardiothoracic surgery. Ann Thorac Surg 2009;88:246-51; discussion 51-2. [Crossref] [PubMed]
- Chalret du Rieu M, Baulieux J, Rode A, et al. Management of postoperative chylothorax. J Visc Surg 2011;148:e346-52. [Crossref] [PubMed]