Endobronchial ultrasound—guidance for interstitial photodynamic therapy of locally advanced lung cancer—a new interventional concept
Introduction
In the past 10 years, convex-probe endobronchial ultrasound (CP-EBUS) has become the procedure of choice for mediastinal lung cancer staging (1). This procedure is widely used for evaluation and diagnosis of mediastinal abnormalities such as lymphoma, sarcoidosis, vascular abnormalities and other tumors (2-4). Recently, the therapeutic role of EBUS is cautiously emerging with few reports of its use in placing fiducial markers for central pulmonary nodules to guide stereotactic body radiation (5,6) and for local injection of chemotherapeutic agents to treat lung cancer (7-10).
Photodynamic therapy (PDT) has been approved for the treatment of non-small cell lung cancer (NSCLC) for over 15 years (11,12). In PDT, systemic administration of a light sensitive drug (i.e., photosensitizer, PS) is followed by illumination of the target tissue with visible light that leads to the generation of reactive oxygen species, notably singlet oxygen (13). This results in the destruction of the tumor by a combination of direct cellular and vascular effects (14). Typically, PDT is accomplished with external beam illumination, or endobronchial photodynamic therapy (EBPDT) (11,12). However, EBPDT is effective in treating cancerous tumors that are smaller than 1 cm (15-18). Interstitial photodynamic therapy (I-PDT) is required to effectively illuminate tumors that are larger or deeper than >1 cm (19). In I-PDT, treatment laser fibers with cylindrical diffuser are inserted into the target tumor through a needle or catheter. The response to I-PDT depends on the light dose that is delivered to the target tumor and margins (20). Image guidance is a key element in administering I-PDT. While intraoperative computed tomography (CT) and standard ultrasound have been used to guide fiber placements for I-PDT to treat other cancers (20,21), CP-EBUS has not been previously described for guiding I-PDT in lung cancer.
In this paper, we used phantom, animal models to describe the use of EBUS with transbronchial needle for the insertion of the laser fibers into tumors. We used computer simulations to demonstrate the propose implementation of this new technique in the treatment of locally advanced lung cancer.
Description of the technique
In vitro evaluation
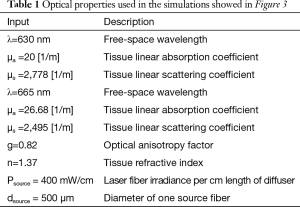
The objective of this experiment was to evaluate the use of the EBUS needle to place the laser cylindrical diffuser in a solid model. Since the mechanical properties of the tumor tissue are most likely to affect the placement, we utilized a solid phantom model that mimics the mechanical properties of soft tissue (Ballistic gel, Clear Ballistics LLC, Fort Smith, AR), as previously described (22). A 21-gauge EBUS needle (NA-201SX-4021; Olympus, Tokyo, Japan) was inserted into the phantom model. After cleaning the EBUS needle tip, the stylet was withdrawn, and a 0.5 mm in diameter optical fiber (RD250 Medlight SA, Ecublens, Switzerland) was passed through the needle. The EBUS needle, with the fiber, was pushed to a distal target region in the phantom. Thereafter, the needle was withdrawn to expose the cylindrical diffuser end of the optical fiber. On the other end, the optical fiber was connected to a diode laser that was used as a light source for delivering laser light from the cylindrical diffuser end to the phantom (Figure 1).
In vivo evaluation
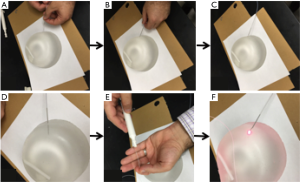
The purpose of this experiment was to test the echogenicity of the laser fiber in vivo. To that end, we conducted a pilot animals study. The Institutional Animal Care and Use Committee (IACUC) at Roswell Park Cancer Institute (RPCI) approved all of the procedures, prior to the experiments.
Five female mice (C3H) bearing SCCVII tumors and two New Zealand White rabbits bearing VX2 tumor were used to assess the feasibility of guiding the RD250 fiber through a 21 gauge EBUS needle (NA-201SX-4021; Olympus, Tokyo, Japan) and 19 gauge needle endoscopic ultrasound (EUS) (Echo-19, Cook Medical, IN, USA), respectively. The linear probe of the ultrasound (Vevo 2100, VisualSonics, Toronto, Canada) system (for the mice) and the curvilinear ultrasound probe of a curved linear array EUS (for the rabbits) were used for real-time ultrasound guidance. Each mouse and rabbit was anesthetized, using isoflurane by inhalation, and placed on a small solid platform in a decubitus position with the tumor upward. The tumor was covered with a water-based gel and the ultrasound probe was placed in contact with the tumor.
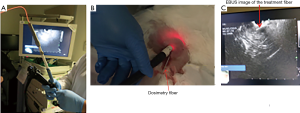
The 21 gauge EBUS needle for the mice and 19-gauge EUS needle for the rabbits was inserted into the tumor under real-time ultrasound guidance. The stylet was withdrawn after cleaning the tip of the needle, and the 0.5 mm in diameter optical fiber (RD250 Medlight SA, Ecublens, Switzerland) was passed through the needle and into the tumor (Figures 2,3). The optical fiber was clearly visualized inside the tumor as shown in Figures 2B,3C.

Proposed implementation
We demonstrated that clinical grade laser fibers with cylindrical diffusers can be inserted through a 21-gauge EBUS needle and 19 gauge EUS needle and can be visualized under real-time ultrasound inside the tumor. We suggest that the I-PDT would be conducted with the clinically approved PS (Porfimer Sodium, Photofrin, Pinnacle Biologics, Chicago, IL), or Chlorin e6, a second generation PS that is associated with very short photosensitivity (1–2 days), and need to be administered only 2–4 h prior to PDT. This PS has shown promising results in the treatment of 42 patients with stage IIIa and IIIb, comparing PDT with Chlorin e6 complex (Radachlorin®; Rada-Pharma, Russia) prior to standard chemotherapy to chemotherapy alone. The neoadjuvant PDT was found to be safe and effective in converting inoperable tumors to operable (23). Another version of this PS (Fotolon, Apocare Pharma, GmbH, Bielefeld, Germany) is being evaluated in a clinical study for NSCLC in Germany (EudraCT ID: 2013-001876-39).
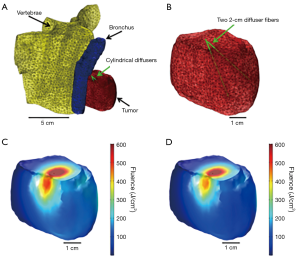
Recent advancements in computer simulations enable near real-time planning and dosimetry, during I-PDT (24-29). We have demonstrated that finite element modelling (FEM) approach can be used to simulate light propagation in geometries that mimic the anatomy of locally advanced tumors (22,24). A detailed description of this approach is provided in Oakley et al. 2015 (24). Briefly, an image visualization and analysis software package (Simpleware, Exeter, UK) is used to segment tumor, adjacent normal tissues, blood vessels and other important anatomical features. The segmented scans are reconstructed to create a 3-dimensional (3-D) model that is imported into a FEM software package (Comsol Inc., Burlington, MA). The FEM, with appropriate boundary and initial conditions is used to calculate the light propagation in the 3-D model. In our laboratory, we produced a simulation that was conducted on a model that was created from de-identified chest CT scan of a patient with locally advanced NSCLC (Figure 4). The de-identified scans were obtained with approval from the RPCI Office of Research Subject Protection. This was a proof of concept simulation to assess the possibility of delivering sufficient light dose to a tumor that could be amenable for EBUS guided I-PDT. The parameters used in this simulation are given in Tables 1,2.
Therefore, we suggest a treatment modality using EBUS guided I-PDT for locally advanced NSCLC, as a new interventional concept that could be considered for evaluation in future phase I/II clinical studies.
Acknowledgements
Funding: This work was supported in part by National Cancer Institute of the National Institutes of Health under Award Number R01 CA193610 to G Shafirstein.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Tremblay A, Stather DR, Maceachern P, et al. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009;136:340-6. [Crossref] [PubMed]
- Grosu HB, Iliesiu M, Caraway NP, et al. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for the Diagnosis and Subtyping of Lymphoma. Ann Am Thorac Soc 2015;12:1336-44. [Crossref] [PubMed]
- Harris K, Modi K, Kumar A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of pulmonary artery tumors: A systematic review (with video). Endosc Ultrasound 2015;4:191-7. [Crossref] [PubMed]
- Harris K, Gomez J, Dhillon S, et al. Convex probe endobronchial ultrasound placement of fiducial markers for central lung nodule (with video). Endoscopic Ultrasound 2015;4:156. [Crossref] [PubMed]
- Argento AC, Decker R, Puchalski J. Fiducial Marker Placement Via Convex Probe EBUS. J Bronchology Interv Pulmonol 2016;23:181-5. [Crossref] [PubMed]
- Khan F, Anker CJ, Garrison G, et al. Endobronchial Ultrasound Guided-Transbronchial Needle Injection (EBUS-TBNI) for Local Control of Recurrent Non-Small Cell Lung Cancer. Ann Am Thorac Soc 2015;12:101-4. [Crossref] [PubMed]
- Harris K, Dhillon SS. Endobronchial ultrasound transbronchial needle injection: ex vivo measurement of dead space volume of the needle assembly. Ann Am Thorac Soc 2015;12:616-8. [Crossref] [PubMed]
- Harris K, Dhillon SS. Reply: Endobronchial Ultrasound Transbronchial Needle Injection: Ex Vivo Measurement of Dead Space Volume of the Needle Assembly. Ann Am Thorac Soc 2015;12:1254. [Crossref] [PubMed]
- Kinsey CM, Garrison G. Endobronchial Ultrasound Transbronchial Needle Injection: Ex Vivo Measurement of Dead Space Volume of the Needle Assembly. Ann Am Thorac Soc 2015;12:1253-4. [PubMed]
- Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst 1998;90:889-905. [Crossref] [PubMed]
- Kato H, Harada M, Ichinose S, et al. Photodynamic therapy (PDT) of lung cancer: experience of the Tokyo Medical University. Photodiagnosis Photodyn Ther 2004;1:49-55. [Crossref] [PubMed]
- Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol 1992;55:145-57. [Crossref] [PubMed]
- Krammer B. Vascular effects of photodynamic therapy. Anticancer Res 2001;21:4271-7. [PubMed]
- Imamura S, Kusunoki Y, Takifuji N, et al. Photodynamic therapy and/or external beam radiation therapy for roentgenologically occult lung cancer. Cancer 1994;73:1608-14. [Crossref] [PubMed]
- McCaughan JS Jr, Williams TE. Photodynamic therapy for endobronchial malignant disease: a prospective fourteen-year study. J Thorac Cardiovasc Surg 1997;114:940-6; discussion 946-7. [Crossref] [PubMed]
- Furuse K, Fukuoka M, Kato H, et al. A prospective phase II study on photodynamic therapy with photofrin II for centrally located early-stage lung cancer. The Japan Lung Cancer Photodynamic Therapy Study Group. J Clin Oncol 1993;11:1852-7. [Crossref] [PubMed]
- Furukawa K, Kato H, Konaka C, et al. Locally recurrent central-type early stage lung cancer < 1.0 cm in diameter after complete remission by photodynamic therapy. Chest 2005;128:3269-75. [Crossref] [PubMed]
- Shafirstein G, Battoo A, Harris K, et al. Photodynamic Therapy of Non-Small Cell Lung Cancer. Narrative Review and Future Directions. Ann Am Thorac Soc 2016;13:265-75. [PubMed]
- Shafirstein G, Bellnier D, Oakley E, et al. Interstitial Photodynamic Therapy-A Focused Review. Cancers (Basel) 2017;9. [Crossref] [PubMed]
- Bown SG, Rogowska AZ, Whitelaw DE, et al. Photodynamic therapy for cancer of the pancreas. Gut 2002;50:549-57. [Crossref] [PubMed]
- Oakley E, Bellnier DA, Hutson A, et al. Surface markers for guiding cylindrical diffuser fiber insertion in interstitial photodynamic therapy of head and neck cancer. Lasers Surg Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Akopov A, Rusanov A, Gerasin A, et al. Preoperative endobronchial photodynamic therapy improves resectability in initially irresectable (inoperable) locally advanced non small cell lung cancer. Photodiagnosis Photodyn Ther 2014;11:259-64. [Crossref] [PubMed]
- Oakley E, Wrazen B, Bellnier DA, et al. A new finite element approach for near real-time simulation of light propagation in locally advanced head and neck tumors. Lasers Surg Med 2015;47:60-7. [Crossref] [PubMed]
- Du KL, Mick R, Busch TM, et al. Preliminary results of interstitial motexafin lutetium-mediated PDT for prostate cancer. Lasers Surg Med 2006;38:427-34. [Crossref] [PubMed]
- Davidson SR, Weersink RA, Haider MA, et al. Treatment planning and dose analysis for interstitial photodynamic therapy of prostate cancer. Phys Med Biol 2009;54:2293-313. [Crossref] [PubMed]
- Axelsson J, Swartling J, Andersson-Engels S. In vivo photosensitizer tomography inside the human prostate. Opt Lett 2009;34:232-4. [Crossref] [PubMed]
- Johansson A, Axelsson J, Andersson-Engels S, et al. Realtime light dosimetry software tools for interstitial photodynamic therapy of the human prostate. Med Phys 2007;34:4309-21. [Crossref] [PubMed]
- Swartling J, Axelsson J, Ahlgren G, et al. System for interstitial photodynamic therapy with online dosimetry: first clinical experiences of prostate cancer. J Biomed Opt 2010;15:058003. [Crossref] [PubMed]