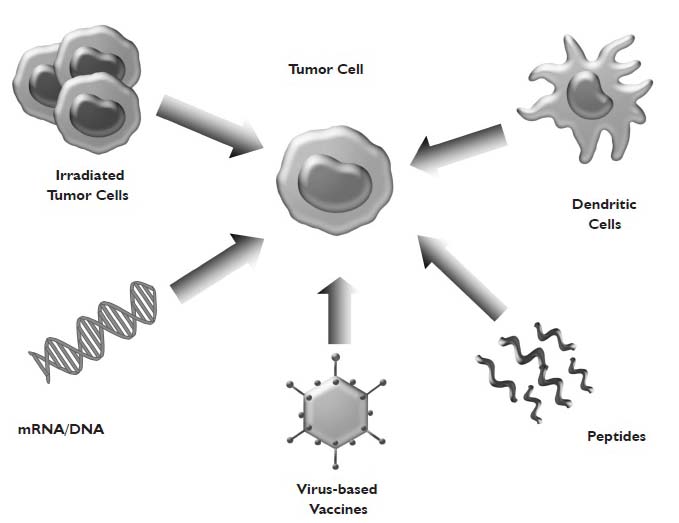
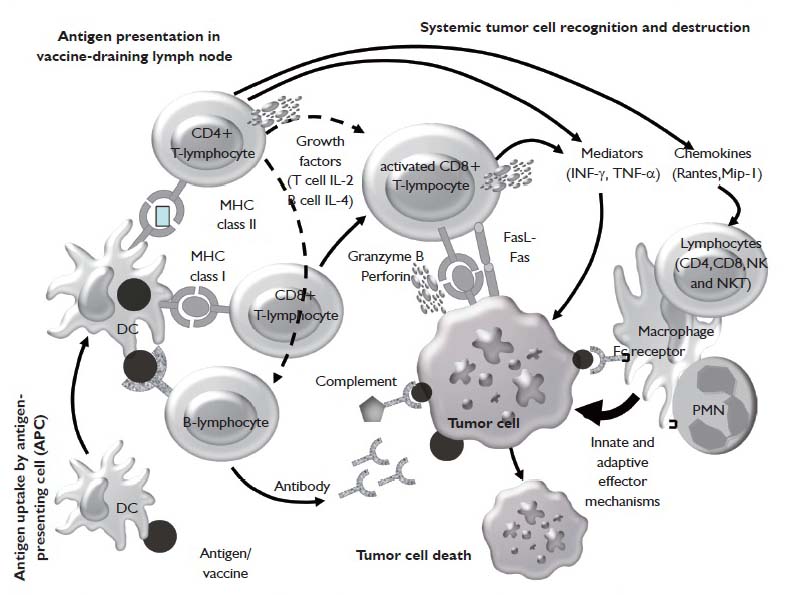
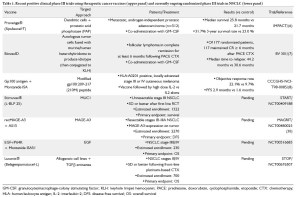
Historically, lung cancer has been regarded as a nonimmunogenic
cancer. Hypotheses as to why active-specific
immunotherapeutic approaches to NSCLC have yielded
disappointing results range from ineffective priming of tumorspecific
T lymphocytes to physical or functional disabling of
immune effector cells by primary host and/or tumor-related
mechanisms. However, there is increasing evidence that NSCLC
and SCLC can evoke specific humoral and cellular antitumor
immune responses. Facilitated methodology for characterizing
antigen profiles in lung cancer may lead to customized
immunotherapy for this disease. Additionally, once molecular
analysis is able to determine more accurately which individuals
are at the highest risk of relapse after surgical resection,
immunotherapy trials may be more efficiently conducted in
a defined population with minimal residual disease in which
immunotherapy will be most likely to provide therapeutic
benefit. Finally, immunotherapeutic approaches in the treatment
of lung cancer will be used in concert with standard treatment
modalities or in combination with multiple immunotherapeutic
agents rather than as single-agent strategies.
|
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin
2010;60:277-300.[LinkOut]
- Thun MJ, Henley S J, Burns D, Jemal A, Shanks TG, Calle EE. Lung cancer
death rates in lifelong nonsmokers. J Natl Cancer Inst 2006;98:691-9.[LinkOut]
- Fossella F, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel
E. Randomized, multinational, phase III study of docetaxel plus platinum
combinations versus vinorelbine plus cisplatin for advanced non-small-cell
lung cancer: the TAX 326 study group. J Clin Oncol 2003;21:3016-24.[LinkOut]
- Scagliotti GV, De Marinis F, Rinaldi M, Crinò L, Gridelli C, Ricci S, et al.
Phase III randomized trial comparing three platinum-based doublets in
advanced non-small-cell lung cancer. J Clin Oncol 2002;20:4285-91.[LinkOut]
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al.
Comparison of four chemotherapy regimens for advanced non-small-cell
lung cancer. N Engl J Med 2002;346:92-8.[LinkOut]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et
al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N
Engl J Med 2010;363:411-22.[LinkOut]
- Schuster SJ, Neelapu SS, Gause BL, Muggia FM, Gockerman JP, Sotomayor
EM, et al. Idiotype vaccine therapy (BiovaxID) in follicular lymphoma
in first complete remission: Phase III clinical trial results. J Clin Oncol
(Meeting Abstracts) 2009;s27:2.
- Schwartzentruber DJ, Lawson D, Richards J, Conry RM, Miller D,
Triesman J, et al. A phase III multi-institutional randomized study of
immunization with the gp100:209-217 (210M) peptide followed by highdose
IL-2 compared with high-dose IL-2 alone in patients with metastatic
melanoma. J Clin Oncol (Meeting Abstracts) 2009;s27:CRA9011.
- Rüttinger D, Winter H, van den Engel NK, Hatz R, Jauch K-W, Fox BA, et
al. Immunotherapy of cancer: key findings and commentary on the third
Tegernsee conference. Oncologist 2010;15:112-8.[LinkOut]
- Morse MA, Whelan M. A year of successful cancer vaccines points to a path
forward. Curr Opin Mol Ther 2010;12:11-3.[LinkOut]
- O’Brien ME, Saini A, Smith IE, Webb A, Gregory K, Mendes R, et al. A
randomized phase II study of SRL172 (Mycobacterium vaccae) combined
with chemotherapy in patients with advanced inoperable non-small-cell lung cancer and mesothelioma. Br J Cancer 2000;83:853-7.[LinkOut]
- O’Brien ME, Anderson H, Kaukel E, O’Byrne K, Pawlicki M, Von Pawel
J, et al. SRL172 (killed Mycobacterium vaccae) in addition to standard
chemotherapy improves quality of life without affecting survival, in patients
with advanced non-small-cell lung cancer: phase III results. Ann Oncol
2004;15:906-14.[LinkOut]
- Stanford JL, Stanford CA, O’Brien ME, Grange JM. Successful
immunotherapy with mycobacterium vaccae in the treatment of
adenocarcinoma of the lung. Eur J Cancer 2008;44:224-7.[LinkOut]
- Dranoff G, Jaffee E, Lazenbay A, Golumbek P, Levitsky H, Brose K, et
al. Vaccination with irradiated tumor cells engineered to secrete murine
granulocyte-macrophage colony-stimulating factor stimulates potent,
specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA
1993;90:3539-43.[LinkOut]
- Salgia R, Lynch T, Skarin A, Lucca J, Lynch C, Jung K, et al. Vaccination
with irradiated autologous tumor cells engineered to secrete granulocytemacrophage
colony-stimulating factor augments antitumor immunity in
some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol
2003;21:624-30.[LinkOut]
- Nemunaitis J, Sterman D, Jablons D, Smith JW 2nd, Fox B, Maples P, et
al. Granulocyte-macrophage colony-stimulating factor gene-modified
autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst
2004;96:326-31.[LinkOut]
- Nemunaitis J, Jahan T, Ross H, Sterman D, Richards D, Fox B, et al.
Phase I/II trial of autologous tumour mixed with an allogeneic GVAX
vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther
2006;13:555-62.[LinkOut]
- Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. Highdose
granulocyte-macrophage colony-stimulating factor-producing
vaccines impair the immune response through the recruitment of myeloid
suppressor cells. Cancer Res 2004;64:6337-43.[LinkOut]
- Rochlitz C, Figlin R, Squiban P, Salzberg M, Pless M, Herrmann R, et al.
Phase I immunotherapy with a modified vaccinia virus (MVA) expressing
human MUC1 as antigen-specific immunotherapy in patients with MUC1-
positive advanced cancer. J Gene Med 2003;5:690-9.[LinkOut]
- Kontani K, Taguchi O, Ozaki Y, Hanaoka J, Sawai S, Inoue S, et al.
Dendritic cell vaccine immunotherapy of cancer targeting MUC1 mucin.
Int J Mol Med 2003;12:493-502.[LinkOut]
- Ramlau R, Quoix E, Rolski J, Pless M, Lena H, Lévy E, et al. A phase II
study of Tg4010 (Mva-Muc1-IL2) in association with chemotherapy
in patients with stage III/IV non-small cell lung cancer. J Thorac Oncol
2008;3:735-44.[LinkOut]
- Acres B, Quoix E, Ramlau R, Lacoste G, Marie Bastien B, Tavernaro A,
et al. Biomarkers associated with clinical outcome in advanced non-small
cell lung cancer patients treated with TG4010. J Clin Oncol (Meeting
Abstracts) 2009;s27:3027.
- Palmer M, Parker J, Modi S, Butts C, Smylie M, Meikle A, et al. Phase I
study of the BLP25 (MUC1 Peptide) liposomale vaccine for active specific
immunotherapy in stage IIIB/IV non-small-cell lung cancer. Clin Lung
Cancer 2001;3:49-57.[LinkOut]
- Butts C, Murray N, Maksymiuk A, Goss G, Marshall E, Soulières D, et al. Randomized Phase IIB trial of BLP25 liposome vaccine in stage IIIB and
IV non-small-cell lung cancer. J Clin Oncol 2005;23:6674-81.[LinkOut]
- Butts C, Maksymiuk A, Goss G, Soulieres D, Marshall E, Cormier Y, et al.
A mulit-centre phase IIB randomized controlled study of BLP25 liposome
vaccine (L-BLP25 or Stimuvax) for active specific immunotherapy of nonsmall
cell lung cancer (NSCLC): updated survival analysis: B1-01. J Thor
Oncol 2007;2:s332-3.[LinkOut]
- Butts C, Murray RN, Smith CJ, Ellis PM, Jasas K, Maksymiuk A, et al. A
multicenter open-label study to assess the safety of a new formulation of
BLP25 liposome vaccine in patients with unresectable stage III non-smallcell
lung cancer. Clin Lung Cancer 2010;11:391-5.[LinkOut]
- Sienel W, Varwerk C, Linder A, Kaiser D, Teschner M, Delire M, et
al. Melanoma associated antigen (MAGE)-A3 expression in stages I
and II non-small cell lung cancer: results of a multi-center study. Eur J
Cardiothorac Surg 2004;25:131-4.[LinkOut]
- Atanackovic D, Altorki NK, Stockert E, Williamson B, Jungbluth AA, Ritter
E, et al. Vaccine-induced CD4+ T cell responses to MAGE-3 protein in
lung cancer patients. J Immunol 2004;172:3289-96.[LinkOut]
- Vansteenkiste J, Zielinski M, Linder A, Dahabre J, Esteban E, Malinowski
W, et al. Final results of a multi-center, double-blind, randomized,
placebo-controlled phase II study to assess the efficacy of MAGE-A3
immunotherapeutic as adjuvant therapy in stage IB/II non-small cell lung
cancer (NSCLC). J Clin Oncol (Meeting Abstracts) 2007;s25:7554.
- Tyagi P, Mirakhur B. MAGRIT: the largest-ever phase III lung cancer trial
aims to establish a novel tumor-specific approach to therapy. Clin Lung
Cancer 2009;10:371-4.[LinkOut]
- Kong F, Jirtle RL, Huang DH, Clough RW, Anscher MS. Plasma
transforming growth factor-ß1 level before radiotherapy correlates with
long term outcome of patients with lung carcinoma. Cancer 1999;86:1712-
9.[LinkOut]
- Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham
C, Cutler J, et al. Phase II study of belagenpumatucel-L, a transforming
growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine
in non-small-cell lung cancer. J Clin Oncol 2006;24:4721-30.[LinkOut]
- Reissmann PT, Koga H, Figlin RA, Holmes EC, Slamon DJ. Amplification
and overexpression of the cyclin D1 and epidermal growth factor receptor
genes in non-small cell lung cancer. Lung Cancer Study Group. J Cancer
Res Clin Oncol 1999;125:61-70.[LinkOut]
- Gonzalez G, Crombet T, Catala M, Mirabal V, Hernández JC, González Y,
et al. A novel cancer vaccine composed of human-recombinant epidermal
growth factor linked to a carrier protein: report of a pilot clinical trial. Ann
Oncol 1998;9:431-5.[LinkOut]
- Gonzalez G, Crombet T, Torres F, Catala M, Alfonso L, Osorio M, et al.
Epidermal growth factor-based cancer vaccine for non-small-cell lung
cancer therapy. Ann Oncol 2003;14:461-6.[LinkOut]
- Ramos TC, Vinageras E, Ferrer MC, Verdecia BG, Rupalé IL, Pérez LM, et
al. Treatment of NSCLC patients with an EGF-based cancer vaccine: report
of a Phase I trial. Cancer Biol Ther 2006;5:145-9.[LinkOut]
- Gonzalez G, Crombet T, Neninger E, Viada C, Lage A. Therapeutic
vaccination with epidermal growth factor (EGF) in advanced lung cancer:
Analysis of pooled data from three clinical trials. Hum Vaccin 2007;3:8-13.[LinkOut]
- Garcia B, Neninger E, de la Torre A, Leonard I, Martínez R, Viada C, et
al. Effective inhibition of the epidermal growth factor/epidermal growth
factor receptor binding by anti-epidermal growth factor antibodies is
related to better survival in advanced non-small-cell lung cancer patients
treated with the epidermal growth factor cancer vaccine. Clin Cancer Res
2008;14:840-6.[LinkOut]
- Neninger Vinageras E, de la Torre A, Osorio Rodriquez M, Catalá Ferrer M,
Bravo I, Mendoza del Pino M, et al. Phase II randomized controlled trial of
an epidermal growth factor vaccine in advanced non-small-cell lung cancer.
J Clin Oncol 2008;26:1452-8.[LinkOut]
- Hirschowitz EA, Foody T, Kryscio R, Dickson L, Sturgill J, Yannelli J, et
al. Autologous dendritic cell vaccines for non-small cell lung cancer. J Clin
Oncol 2004;22:2808-15.[LinkOut]
[LinkOut]
- Hirschowitz EA, Foody T, Hidalgo GE, Yannelli JR. Immunization of
NSCLC patients with antigen-pulsed immature autologous dendritic cells.
Lung Cancer 2007;57:365-72.[LinkOut]
- Hirschowitz EA, Mullins A, Prajapati D, Baeker T, Kloecker G, Foody T, et
al. Pilot study of 1650-G: A Simplified Cellular Vaccine for Lung Cancer. J
Thorac Oncol 2011;6:169-73.[LinkOut]
- Morse MA, Clay TM, Hobeika AC, Osada T, Khan S, Chui S, et al. Phase I
study of immunization with dendritic cells modified with fowlpox encoding
carcinoembryonic antigen and costimulatory molecules. Clin Cancer Res
2005;11:3017-24.[LinkOut]
- Kelly RJ, Gulley JL, Giaccone G. Targeting the immune system in nonsmall-
cell lung cancer: bridging the gap between promising concept and
therapeutic reality. Clin Lung Cancer 2010;11:228-37.[LinkOut]
- Barve M, Bender J, Senzer N, Cunningham C, Greco FA, McCune D, et
al. Induction of immune responses and clinical efficacy in a phase II trial
of IDM-2101, a 10-epitope cytotoxic T-lymphocyte vaccine, in metastatic
non-small-cell lung cancer. J Clin Oncol 2008;26:4418-25.[LinkOut]
- Morris JC, Vahanian N, Janik JE, Moses L, Tennant L, Pittaluga
S, et al. Phase I study of an antitumor vaccination using α-(1,3)
galactosyltransferase expressing allogeneic tumor cells in patients with
refractory or recurrent non-small cell lung cancer (NSCLC). J Clin Oncol
(Meeting Abstracts) 2005;s23:2586.
- Raez LE, Cassileth PA, Schlesselmann JJ, Sridhar K, Padmanabhan S,
Fisher EZ, et al. Allogeneic vaccination with a B7.1 HLA-A gene-modified
adenocarcinoma cell line in patients with advanced non-small-cell lung
cancer. J Clin Oncol 2004;22:2800-7.[LinkOut]
- Neninger E, Diaz RM, de la Torre A, Rives R, Díaz A, Saurez G, et al. Active
immunotherapy with 1E10 anti-idiotype vaccine in patients with small cell
lung cancer: report of a phase I trial. Cancer Biol Ther 2007;6:145-50.[LinkOut]
- Ma J, Poehlein CH, Jensen SM, LaCelle MG, Moudgil TM, Rüttinger D, et
al. Manipulating the host response to autologous tumor vaccines. Dev Biol
(Basel) 2004;116:93-107.[LinkOut]
- Dudl e y ME, Wunde r l i ch JR , Robbins PF, Yang JC, Hwu P,
Schwartzentruber DJ, et al. Cancer regression and autoimmunity in
patients after clonal repopulation with antitumor lymphocytes. Science
2002;298:850-4.[LinkOut]
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al.
Adoptive cell therapy for patients with metastatic melanoma: evaluation of
intensive myeloablative chemoradiation preparative regimens. J Clin Oncol
2008;26:5233-9.[LinkOut]
- Rüttinger D, van den Engel NK, Winter H, Schlemmer M, Pohla H,
Grützner S, et al. Adjuvant therapeutic vaccination in patients with
non-small cell lung cancer made lymphopenic and reconstituted with
autologous PBMC: first clinical experience and evidence of an immune
response. J Transl Med 2007;5:43.[LinkOut]
Cite this article as: Winter H, van den Engel NK, Rusan M, Schupp N, Poehlein CH, Hu HM, Hatz RA, Urba WJ, Jauch KW, Fox BA, Rüttinger D. Active-specific immunotherapy for non-small cell lung cancer. J Thorac Dis 2011;3(2):105-114. doi: 10.3978/j.issn.2072-1439.2010.12.06
|