Cluster analysis of acute ascending aortic dissection provides novel insight into mechanisms of distal progression
Introduction
The DeBakey classification of aortic dissection was first described by the Houston group in 1965 (1) where type I involved the ascending aorta and progresses into the descending aorta. DeBakey type II dissections involve the ascending aorta only without distal progression. While the anatomical distinction was clear, both warranted emergent surgical intervention based on involvement of the ascending aorta and a natural history associated with high mortality. The reasons for the difference in distal progression of aortic dissection remain unclear. Indeed, the Stanford classification (2) consolidates DeBakey type I and II dissections into the type A category based on the therapeutic paradigm that emergency surgery is necessary to lower early mortality in dissections that involve the ascending aorta. In contrast, Stanford Type B and its corollary DeBakey type III dissections typically do not require surgical intervention in the absence of complications (3).
Majahalme et al. from the International Registry of Acute Aortic Dissections (IRAD) described type I dissection patients as being older with a higher incidence of acute renal failure and more likely to have findings of distal malperfusion. In-hospital and 5-year survival were not different between type I and II dissections (4). As expected, distal aortic interventions are more likely with younger age, Marfan syndrome, and DeBakey type I dissections (5-7). Tsagakis et al. had previously suggested an extension of the DeBakey type II classification to include the proximal and mid arch (8).
To better understand the morphology of the dissected aorta and its relationship to primary tear size, we performed measurements from reconstructed three-dimensional computerized tomographic (CT) images throughout the length of the aorta. From these measurements we used statistical clustering techniques to obtain a summative categorization of acute ascending aortic dissections. We then examined the differences in primary tear size, other morphological parameters, and clinical outcomes.
Methods
Patients
This study was approved by the University of Wisconsin-Madison Institutional Review Board (No. 2015-1050). A waiver of the need to obtain consent from patients was approved. Records from 108 consecutive patients with available preoperative aortic images who underwent repair of acute type A aortic dissection at the University of Wisconsin Hospitals and Clinics between January 2000 to February 2016 were analyzed. Ascending dissection was defined as acute if the onset of symptoms was less than 14 days from the time of surgery. Diagnosis was made by CT scan, echocardiography and surgical findings.
Imaging analysis
Three-dimensional reconstruction of CT scan images was performed using TeraRecon iNtuition image analysis software (TeraRecon Inc., Foster City, CA, USA). Aortic measurements were made using a center-line method which yields aortic cross-sectional images orthogonal to the direction of blood flow (9). This avoids inaccurate measurements from inadvertent oblique images. CT scan measurements were performed by a faculty cardiothoracic surgeon with expertise in evaluations and operative intervention for aortic disease.
For the true and false lumen, we measured the luminal areas, and fraction of the total perimeter that the true and false lumen occupies to quantify the degree of circumferential medial separation or injury. False lumen flow is quantified in the arterial phase of the CT scan as the contrast intensity ratio of the false lumen to that of the true lumen. We also noted the location and size of the primary and secondary tears. The estimated area of the primary tear was calculated by multiplying the length by the width of the tear. We also corroborated the size and location of the tears with intraoperative findings on transesophageal echocardiography and gross anatomy.
We noted the presence of dissection of non-coronary aortic branches including the innominate, right carotid, right subclavian, left carotid, and left subclavian arteries. Translational motion artifact at the aortic root prevented accurate in-vivo imaging of coronary perfusion.
Follow up
Survival data was available for all 108 patients. Mid-term survival data was obtained through detailed clinical follow-up. The maximum follow up was 13.3 years with a total follow-up of 277.9 patient years and a median follow-up of 1.30 years (IQR =3.45).
Statistical methods
A two-step cluster analysis was performed on true lumen to total aortic area ratio at the level of the aortic root, mid-ascending aorta, proximal arch, distal arch, mid-descending thoracic aorta, and abdominal aorta. This technique categorized type A dissections based on true to total lumen area ratio along the entire length of the aorta. Pearson chi square test or Fisher’s exact test was used to analyze categorical variables. Kaplan-Meier survival curve with Mantel-Cox statistics were used to analyze survival data. Student’s t-test was used to compare continuous variables. Statistics including two-step clustering were performed using Statistical Package for the Social Sciences software (SPSS Inc., Chicago, IL, USA).
Results
Cluster analysis of acute aortic dissection involving the ascending aorta
Two-step cluster analysis resulted in 2 distinct acute ascending dissection groups (Table 1) with an excellent degree of separation (silhouette cluster of cohesion and separation =0.6). The first cluster corresponds to DeBakey type I dissection (n=71) where the aorta is dissected from the ascending to past the distal arch. In contrast, the second cluster (Table 1) describes a dissection that extends from the ascending aorta to the distal arch. This corresponds to an “extended” DeBakey type II dissection (n=37). This suggests that some degree of dissection involving the entire aortic arch is a frequent phenomenon that occurs without more extensive distal descending aortic progression.

Full table
Characteristics of extended DeBakey type II dissections
Extended type II dissections had primary tears that were nearly twice the area of type I dissections (6.57±1.00 vs. 3.72±0.33 cm2, P=0.009). The primary tear location in type I vs. extended type II are as follows: root [16 (22.5%) vs. 7 (18.9%), P=0.7)], ascending aorta [37 (52.1%) vs. 22 (59.5%), P=0.467], proximal arch [7 (9.9%) vs. 7 (18.9%), P=0.2)], distal arch [6 (8.5%) vs. 1 (2.7%), P=0.3)], and descending thoracic aorta [5 (7.0%) vs. 0 (0%), P=0.1]. In extended type II dissections, there were a lower average number of secondary tears (0.54±0.96 vs. 1.90±1.6, P<0.001), and smaller combined secondary tear diameters (0.76+1.36 vs. 2.11+1.96 cm, P<0.001) likely due to the much shorter length of aorta involved. There were no differences in the total diameter (0.53±1.01 vs. 0.66±1.14 cm, P=0.5) or number (0.37±0.62 vs. 0.41+0.60, P=0.8) of secondary re-entry tears in the arch for type I and extended II respectively (P>0.1).
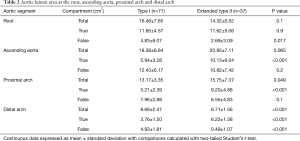
Similar to findings by Sandhu et al. (10), we found that extended type II dissections had a larger total aortic area at the ascending aorta (P=0.065) and proximal arch (P=0.049) levels (Table 2). Extended type II dissection also had a true to total lumen area ratio (Table 1) that was higher in the ascending aorta (P<0.01) and proximal arch (P<0.001) with a trend towards this at the root level (P=0.1). This corresponded to a larger absolute true lumen area (Table 2) at the ascending aorta and proximal arch levels (P<0.001). The root in extended type II dissections had a smaller false lumen area (P=0.017, Table 2) suggesting less sinus wall injury.
Full table
The true lumen to total lumen cross sectional perimeter ratio (i.e., circumferential portion of the aortic wall without dissection) is greater in extended type II dissections at the ascending aorta (0.40±0.21 vs. 0.30±0.16, P=0.016), proximal arch (0.45±0.30 vs. 0.27±0.17, P<0.01) and with a trend at the root level (0.70±0.27 vs. 0.63±0.26, P=0.1). This suggests less circumferential aortic wall delamination at the root, ascending and proximal arch level. There was no difference in false lumen to true lumen contrast intensity ratio at the root (0.55±0.42 vs. 0.49±0.42, P=0.5) and ascending aorta (0.69±0.33 vs. 0.62±0.34, P=0.4) but becomes significantly lower in the extended type II group at the proximal (0.51±0.38 vs. 0.70±0.33 P<0.01) and distal arch levels (0.11±0.07 vs. 0.67±0.34, P<0.001) indicating sluggish flow or thrombosis at these sites as the dissected aortic layers begin to reconstitute into a single layer. In type I dissections, 9.6% extended to the distal descending thoracic aorta, 27% extended to the celiac aorta, 1.9% extended to the infrarenal aorta and 61.5% reached the iliac vessels. All type I dissections extended beyond the mid-thoracic aorta. In extended type II dissections, 13.5% were limited to the ascending aorta as originally defined by Debakey et al. (1), 78.4% reached the proximal and transverse arch, and 8.1% reached the distal arch.
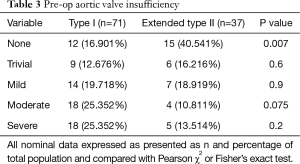
Interestingly, the aortic annulus is larger in type I than in extended type II (4.60±1.27 vs. 4.02±1.26 cm2, P=0.025). Considering that there is less aortic root injury in extended type 2 dissections, this may explain the lower degree of aortic insufficiency seen on preoperative echocardiography (Table 3). We also examined the average number of dissected arch branches (i.e., innominate, right and left carotid, right and left subclavian) and found that extended type II dissections had much lower number of arch branch dissections per patient (1.67±1.67 vs. 0.76±1.32, P=0.003). Interestingly, type I dissections had a lower diastolic and mean blood pressure on initial presentation which may reflect the greater number of arch vessel dissections in these patients (Table 4).
Full table
Full table
Patient demographics and operative parameters
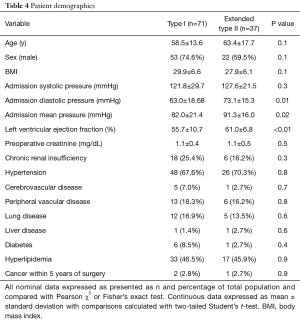
There were no differences in patient age, gender, body mass index, preoperative creatinine, or other major comorbidities with the exception of coronary artery disease (Table 4). Atherosclerotic coronary artery disease is defined as greater than >50% stenosis of the left main coronary and/or >70% stenosis of other coronary vessels. There was a greater incidence of prior coronary artery bypass grafting (CABG) and previous percutaneous coronary interventions (PCI) with more diseased coronary vessels in the extended type II population (Table 5). We reviewed the medical record to confirm that the acute dissection did not result as a complication of recent CABG. There was a greater incidence of coronary artery disease in the extended type II population, but we did not see any differences in a history of peripheral vascular or cerebrovascular disease (Table 4). Alternatively, although not statistically significant, the greater age in the extended type II group may also explain more coronary artery disease (58.5±13.6 vs. 63.4±17.7 years, P=0.1). The left ventricular ejection fraction was lower the in the type I group (55.7±10.7% vs. 61.0±6.8%, P<0.01) despite a lower incidence of coronary disease perhaps due to acute strain placed on the heart due to the smaller true to total lumen ratio and a larger false lumen area in the distal aorta leading to flow obstruction (Table 4).
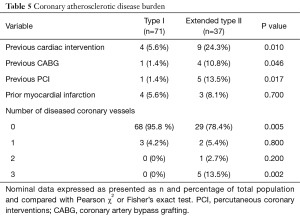
Full table
There were no differences (P>0.05) in operative year, prior cardiac surgery, operative strategies for the aortic valve, extent of aortic replacement, cardiopulmonary bypass and aortic cross clamp times, duration of circulatory arrest, and cerebral protection strategies used (Table S1). There were no differences (Table S2) in postoperative complications (P>0.05) or length of postoperative hospital stay (P=0.4).
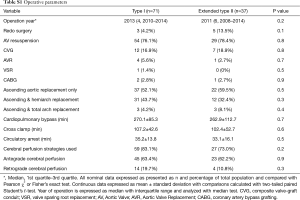
Full table
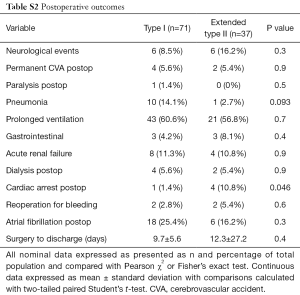
Full table
Patient presentation, malperfusion, and survival
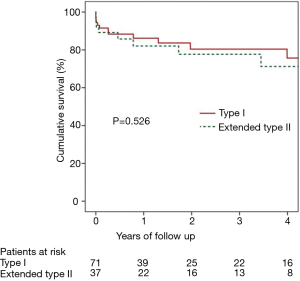
As expected from distal dissection propagation, type I dissection patients presented more frequently with abdominal and lower extremity pain (P<0.05), and malperfusion syndromes (P<0.01) (Table S3). There was no difference in mean follow-up between type I and extended type II groups (2.4±3.0 vs. 2.8±3.3 years, P=0.5). There were more distal aortic and peripheral vascular interventions for dissection flap-related complications (Table S3) in type I dissections (9.9% vs. 0%, P<0.05). However, more distal interventions are expected with longer follow-up. Distal interventions included two open repairs for distal thoracoabdominal dissecting aneurysms, one TEVAR for a descending thoracic aortic aneurysm, one TEVAR for a patient with severe back pain which occurred with hypertensive episodes, one patient underwent renal artery stenting for dissection flap obstruction of renal ostia, and two patients had operative interventions for lower extremity ischemia. There were no differences between type I and extended type II dissections (Figure 1) respectively in 30-day (8.5% vs. 10.8%, P=0.7) and 5-year mortality (24.3% vs. 28.8%, P=0.5).
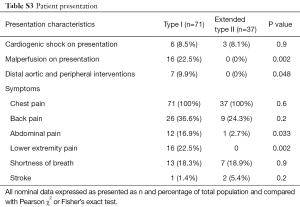
Full table
Discussion
Our studies reveal several clues as to the etiology for the development of a type I versus an “extended” type II dissection (Figure 2). The finding that the primary tear area in extended type II dissections is almost twice the size of that in type I is unexpected. It is possible that the higher diastolic and mean blood pressure in the extended type II group lead to a larger primary tear. However, the small blood pressure difference of about 10 mmHg measured in the upper extremity more likely reflects the lower incidence of arch vessel dissection in this group. The larger primary tear in extended type II dissections may enable the false lumen to self-decompress though the primary tear itself such that the false lumen never developed enough intraluminal pressure or energy for more extensive distal propagation. Tsai et al. performed an ex vivo model that examined fluid dynamics in various dissection scenarios as determined by relative primary tear size and whether an exit tear was present (11). Their studies demonstrated a lower pressure generated in the false lumen in a larger primary tear compared with that of a smaller tear. This difference in false lumen pressure occurs particularly when there not a re-entry tear which is the case when the dissection is first initiated from the primary tear site (11). There is likely a time lag in the development of the secondary re-entry tear after the start of dissection propagation from the primary tear.

An alternative explanation is that there an inherent difference in the aortic delamination strength in type I versus extended type II dissections such that without sufficient energy to extend the delamination process distally, the false lumen pressure builds leading to enlargement of the primary tear. Differences in lamination strength of the aorta have been previously described by Pasta et al. (12). Interestingly, the limited delamination in extended type II dissection not only occurs lengthwise but also applies along the perimeter so more of the aortic wall is preserved in a circumferential direction. Future studies into the delamination strength of type I versus extended type II dissection aortic walls may clarify this issue. The greater incidence of coronary artery disease in the extended type II dissection group suggests a greater vascular atherosclerotic burden.
The limited distal propagation in extended type II dissection corresponds to less arch branch dissections relative to type I. With the “extended” DeBakey type II definition, this group is unlikely to need distal aortic intervention as long as the primary tear is resected in the primary operation whether that is accomplished by ascending aortic replacement alone, hemi-arch or total arch replacement. With DeBakey type I dissections, the likelihood of late distal aortic interventions ranges from 6% to 15% (13-15). Our results demonstrate that it is very unusual for a dissection to stop at the level of the mid-thoracic aorta as all type I dissections progressed beyond this point.
The DeBakey classification is extremely useful in determining surgical urgency and the need for future interventions. However, the Stanford type A and B classification focuses on the surgical emergency of the ascending dissection component. This has been deeply ingrained not only amongst surgeons but also in the vocabulary of internists, emergency medicine physicians and other non-surgeon physicians whose role is to facilitate timely diagnosis and transfer of patients with ascending dissections but rarely is faced with managing residual descending aortic dissections. Thus the role of the Stanford classification is expected to remain very relevant.
With the advent of novel primary surgical strategies to manage the descending thoracic aorta in acute ascending aortic dissection (e.g., frozen elephant trunk, proximalization of arch vessels), a more in-depth understanding of the anatomic, mechanistic, and clinical differences between DeBakey type I and type II dissection becomes even more relevant. While the classic definition of a DeBakey type II dissection describes an aortic delamination process that involves only the ascending aorta (1), our data using a statistical categorization technique suggests that clinical presentation and outcomes are similar even when the dissection ends more towards the distal arch. The dissection often extends across the transverse aortic arch into the distal arch. Although we do find a false lumen in the distal arch, it tends to be small and often has low flow or is thrombosed, and rarely causes long-term sequelae.
Clustering is an exploratory statistical method that may reveal novel groupings within a dataset (16). In our study, it revealed patterns of dissection morphology which may not have been previously appreciated based on experience accumulated through visual assessment alone. While the statistical clustering of dissection anatomical features does not take into account the therapeutic implications, disease anatomy often contributes to clinical presentation and therapeutic options, perhaps especially so in the aorta. Therefore, we believe statistical categorization of diseases parameters may be a reasonable method to discover otherwise under-appreciated pathological patterns.
Tsagakis et al. previously described a modified DeBakey type II dissection that “stops at the left subclavian artery with freedom from a false lumen in the descending aorta” (8). Our statistical categorization is consistent with this observation but also suggests an extension of this definition past the left subclavian and into the distal arch. This group of extended type II dissections have a similar clinical phenotype with no symptoms attributable to distal malperfusion (e.g., abdominal or lower extremity pain), no need for intervention in the descending aorta or its branches, and tends to have less aortic valve insufficiency.
The findings in this study are limited by its retrospective nature in a single institution with its inherent limitations and biases. Due to variation in the quality of the CT scans, we only included studies of adequate quality in the form of arterial phase CT scans with a resolution that defined the aortic anatomy adequately for our measurements. The mean follow-up of 2.6 years was relatively short and we would expect more distal aortic interventions in type I dissections on longer follow-up. Branch perfusion, especially in the acute phase, is dynamic so relative perfusion by the true and false lumen is likely not fully appreciated on a static image. However, static CT scan images are currently the most widely accessible imaging technique for diagnosis.
Statistical clustering or categorization techniques based on disease morphology and characteristics may be a useful screening tool in the development of cardiovascular disease classifications that ultimately should guide therapeutic options or have prognostic significance. Application of this mathematical technique to other disease processes may be useful in helping us understand human pathology, particularly in large datasets such as population disease registries.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the University of Wisconsin-Madison Institutional Review Board (No. 2015-1050). A waiver of the need to obtain consent from patients was approved.
References
- Debakey ME, Henly WS, Cooley DA, et al. Surgical Management of Dissecting Aneurysms of the Aorta. J Thorac Cardiovasc Surg 1965;49:130-49. [PubMed]
- Daily PO, Trueblood HW, Stinson EB, et al. Management of acute aortic dissections. Ann Thorac Surg 1970;10:237-47. [Crossref] [PubMed]
- Charilaou P, Ziganshin BA, Peterss S, et al. Current Experience With Acute Type B Aortic Dissection: Validity of the Complication-Specific Approach in the Present Era. Ann Thorac Surg 2016;101:936-43. [Crossref] [PubMed]
- Majahalme N, Kohl L, Masip AE, et al. DeBakey Types I and II are distinct subsets within type A dissection: A report from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2013.61.
- Kirsch M, Soustelle C, Houel R, et al. Risk factor analysis for proximal and distal reoperations after surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2002;123:318-25. [Crossref] [PubMed]
- Moore NR, Parry AJ, Trottman-Dickenson B, et al. Fate of the native aorta after repair of acute type A dissection: a magnetic resonance imaging study. Heart 1996;75:62-6. [Crossref] [PubMed]
- Geirsson A, Bavaria JE, Swarr D, et al. Fate of the residual distal and proximal aorta after acute type a dissection repair using a contemporary surgical reconstruction algorithm. Ann Thorac Surg 2007;84:1955-64; discussion-64.
- Tsagakis K, Tossios P, Kamler M, et al. The DeBakey classification exactly reflects late outcome and re-intervention probability in acute aortic dissection with a slightly modified type II definition. Eur J Cardiothorac Surg 2011;40:1078-84. [PubMed]
- Rengier F, Weber TF, Giesel FL, et al. Centerline analysis of aortic CT angiographic examinations: benefits and limitations. AJR Am J Roentgenol 2009;192:W255-63. [Crossref] [PubMed]
- Sandhu HK, Charlton-Ouw KM, Rice RD, et al. Natural History and Management of DeBakey Type II Aortic Dissection. American Association for Thoracic Surgery Annual Meeting 2016. Available online: http://webcast.aats.org/2016/Video/Tuesday/05-17-16_Ballroom_I_1631_Sandhu-800.mp4;http://webcast.aats.org/2016/Detail.php?d=Tuesday&s=10
- Tsai TT, Schlicht MS, Khanafer K, et al. Tear size and location impacts false lumen pressure in an ex vivo model of chronic type B aortic dissection. J Vasc Surg 2008;47:844-51. [Crossref] [PubMed]
- Pasta S, Phillippi JA, Gleason TG, et al. Effect of aneurysm on the mechanical dissection properties of the human ascending thoracic aorta. J Thorac Cardiovasc Surg 2012;143:460-7. [Crossref] [PubMed]
- Rylski B, Milewski RK, Bavaria JE, et al. Long-term results of aggressive hemiarch replacement in 534 patients with type A aortic dissection. J Thorac Cardiovasc Surg 2014;148:2981-5. [Crossref] [PubMed]
- Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;133:127-35. [Crossref] [PubMed]
- Rylski B, Beyersdorf F, Kari FA, et al. Acute type A aortic dissection extending beyond ascending aorta: Limited or extensive distal repair. J Thorac Cardiovasc Surg 2014;148:949-54; discussion 54. [Crossref] [PubMed]
- Jain AK, Murty MN, Flynn PJ. Data clustering: A review. Acm Computing Surveys 1999;31:264-323. [Crossref]