Triaging patients with left main disease after the EXCEL and NOBLE trials: the everlasting saga of coronary artery bypass grafting and percutaneous coronary intervention
The left main (LM) coronary artery supplies a large portion of the ventricular myocardium and its disease poses important decisional challenges. Based on European guidelines, LM revascularization must be undertaken whenever a lesion is angiographically >50% in presence of documented ischemia or fractional flow reserve ≤0.80 (1). The same guidelines clarify that the benefit of LM revascularization for these patients is not just a matter of symptoms relief, but a matter of prognosis. In fact, LM disease may manifest not only as stable angina but also as a large myocardial infarction (MI) or sudden death.
Coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) are valid alternatives to revascularize a diseased LM, and advocates of both strategies have convincing arguments on their side (2). CABG, the historical gold standard, is intuitively advantageous in the frequent circumstances when the plaque is located at the level of the LM bifurcation or when other stenoses are found downstream in the left vascular territory. Indeed, the long-standing protective effect of an arterial bypass on a long segment of the left anterior descending coronary artery is unmatched by PCI, which addresses only the treated lesion and cannot prevent the effect of atherosclerotic progression in other coronary segments. On the other hand, PCI of the LM is a relatively quick procedure in expert hands, more cumbersome but feasible when treating the bifurcation requires more than one stent, and it is minimally invasive compared with CABG thus allowing for quicker hospital recovery and discharge. All things being equal, most patients with LM disease in need of revascularization would prefer PCI. But are all things equal when PCI and CABG are compared on the ground of early and late safety and efficacy? To answer this question one has to look at head-to-head randomized clinical studies and meta-analyses comparing these treatment options.
In 2011, a meta-analysis of 1,611 LM patients from three randomized clinical trials and a post-hoc analysis of a randomized clinical trial compared PCI and CABG with respect to their clinical outcomes at 1 year (3). The two largest studies available at that time were SYNTAX (the study cohort of SYNTAX actually included also patients with three-vessel and no LM disease) and PRECOMBAT, where CABG was compared versus PCI with first-generation drug-eluting stents (4,5). In the pooled analysis, PCI was associated with a non-significantly 28% higher risk of major adverse cardiac or cerebrovascular events [MACCE, a composite of death, MI, stroke or target vessel revascularization (TVR)], a statistically significant increase in TVR and a decrease in stroke. No statistically significant differences were noted with respect to death and MI. These findings were substantially replicated in a more recent and larger meta-analysis comprising 14,203 patients from 24 studies (mostly observational), with no evidence for increased mortality with PCI up to 5 years, but a long-lasting risk of higher TVR compared with CABG, and less stroke (6).
In 2016, two new trials of PCI vs. CABG for LM revascularization have been published by the EXCEL and NOBLE investigators (7,8). The conclusions of these trials sound discordant (i.e., PCI was non-inferior to CABG in EXCEL, whereas CABG was better than PCI in NOBLE), but are largely explained by disparities in study endpoints (i.e., EXCEL did not incorporate TVR in the primary composite endpoint, and used an MI definition that included periprocedural events), time of assessment (3 years in EXCEL, 5 years in NOBLE) and procedural characteristics (everolimus-eluting stents were used in EXCEL, biolimus-eluting stents were mostly used in NOBLE) (9). Compared with previous LM trials, patients from EXCEL and NOBLE were more selected (i.e., those with broader anatomical complexity were excluded) and procedure characteristics in the PCI and CABG arms were more reflective of current practice standards (10).
With about 3,000 additional randomized patients from EXCEL and NOBLE, there is clearly an opportunity for updating existing meta-analyses. This effort has been recently undertaken by Khan and colleagues, who combined a total of 4,700 patients from the five available LM trials and the post-hoc analysis of SYNTAX (11). This sample cumulatively reflects a LM population with distal or bifurcation disease in 68%, multivessel disease in 64% and low-to-intermediate SYNTAX score in 78%. This has implications for the generalizability of the study findings because patients with high SYNTAX score were poorly represented. As expected by the low-to-intermediate burden of atherosclerosis, the desirable goal of complete revascularization was met in a relatively high proportion of patients (84–86%) in both arms of the meta-analysis. Among the five studies that reported early (0 to 1 year) outcomes, non-significant differences were noted in MACCE and the composite of death, MI or stroke. Similarly, there were no differences in mortality and spontaneous MI. Stroke was 64% significantly lower in patients undergoing PCI, and revascularization increased by 84%. It should be noted that the results of meta-analyses for MACCE and revascularization were significantly heterogeneous mostly due to the inclusion of the EXCEL trial, although removal of this study did not unduly affect the direction of the effect estimate. Among the four studies that reported late (>3 years) outcomes, the results were consistent for MACCE and the composite of death, MI or stroke, with NOBLE acting as the most influential study (although, again, no major deviations of the effect estimate occurred after study removal). There were also no differences in death, MI or stroke taken in isolation, while repeat revascularization remained largely higher in the PCI group. With respect to spontaneous MI, the exclusion of EXCEL resulted in a significant long-term increase in patients undergoing PCI. NOBLE was the major determinant for the heterogeneity in the treatment effect for stroke, and the reasons why PCI was associated with more stroke than CABG in that trial remains unclear. Khan et al. also attempted a meta-analysis stratified by stent type and did not find a significant interaction between the generation of drug-eluting stent used in the PCI arm (i.e., first- vs. second-generation) and the outcome of repeat revascularization. In contrast, a significant interaction was found between MACCE and SYNTAX score category (i.e., low-to-intermediate vs. high) at >3 years, meaning that current guidelines excluding PCI for patients with high SYNTAX score are justified (1).
Indeed, when looking at the results of the meta-analysis from Khan et al., it seems that new data from EXCEL and NOBLE have not altered too much the state of the art. In fact, the Achilles’ heel of PCI for LM disease remains TVR. This sounds disappointing as it tones down the emphasis on procedural improvements occurred over the last decade in the PCI field (i.e., advent of second-generation drug-eluting stents, more extensive use of intravascular imaging and fractional flow reserve guidance). While repeat revascularization remains a non-negligible outcome, however, its consequences are less prone to irreversible harm compared to death, MI or stroke. Weighing TVR as much as other harder endpoints overly penalize PCI when MACCE is chosen as the primary endpoint and time-to-first-event computation is performed (12). In spite of the significant increase in revascularization, the understanding that the composite of death, spontaneous MI or stroke is not significantly different between PCI and CABG both at 1 year and >3 years is partly reassuring, but this result needs confirmation at long-term follow-up. In fact, in EXCEL, the Kaplan-Meier curves for the primary endpoint of death, MI or stroke separated early in favor of PCI due to more periprocedural MI in the CABG group, but then converged up 3 years until a point of superimposition (7). This makes longer follow-up observations of this trial required to ascertain whether CABG has truly time-dependent benefits that would make the overall interpretation more problematic for PCI.
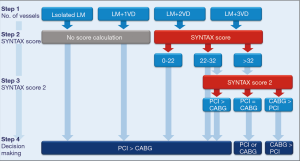
Can we use risk scores to triage LM patients to PCI or CABG? Clinical guidelines endorse using the SYNTAX score based on its ability to categorize patients at high risk of adverse events following PCI, and the SYNTAX score 2 based on its ability to guide decision-making for PCI or CABG with the inclusion of clinical factors on top of anatomic considerations (1). Yet, these scores have caveats not just limited to their inter-observer and intra-observer variability, or the underestimation of lesion complexity as compared with core-lab assessment (13). In EXCEL and NOBLE, the SYNTAX score failed to separate meaningfully the outcomes of PCI and CABG across risk categories, which questions its accuracy in patients at lower risk (7,8). In an external validation study of the SYNTAX score 2, the calibration for 4-year mortality was good, with the exception of patients with high predicted mortality after PCI, who experienced less mortality than expected (14). In another validation study using patients from PRECOMBAT and the post-hoc analysis of SYNTAX, calculating the SYNTAX score 2 resulted in predicted equipoise of PCI and CABG for 74% of patients, which was confirmed in the actual follow-up of death at 5 years (15). When the score predicted PCI as the best option, the true outcomes went in the expected direction, with higher rates of 5-year mortality in the CABG arm. However, this was not the case when CABG was predicted as the best option because in these patients the mortality rates were actually similar between patients who underwent PCI or CABG (15). Therefore, the SYNTAX score and the SYNTAX score 2 may serve as useful criteria to guide the Heart Team discussion on a less subjective basis, but attention should be paid on avoiding these criteria to be the only metrics for decision-making on the ground of prognostic considerations, particularly when CABG is selected as the best option by the SYNTAX score 2. All in all, it seems reasonable to calculate the SYNTAX score of patients with LM and multivessel disease (i.e., those with isolated LM or LM plus single vessel disease virtually have a low or intermediate SYNTAX score) to identify good candidates to PCI (Figure 1). Refinements in risk stratification with the SYNTAX score 2 may prove useful for patients with multivessel disease in the intermediate- and high-risk categories to identify other potential candidates to PCI. In general, CABG remains the preferable option when complete revascularization by means of PCI is not achievable or achievable at the price of complex interventions and too many stents implanted (Figure 1).
Finally, what information should a contemporary patient with LM disease receive from the Heart Team at the time of consenting to revascularization? Based on the updated meta-analysis of Khan et al., patients should be aware that PCI and CABG compare similarly up to 3 years with respect to hard clinical outcomes if the complexity of atherosclerosis extending beyond the LM is not too high. Patients with more extensive atherosclerosis beyond the LM are possibly best addressed by CABG, but whether this also occurs when TVR is removed from the equation will remain unclear until long-term follow-up of EXCEL is completed. Yet, some patients may weigh favorably the increased risk of repeat revascularization with PCI against the longer hospital stay and recovery of CABG. Other may value the long-lasting protective effect of an arterial bypass grafting more than the minimally invasive nature of PCI. The paradigm of the Heart Team directing patients to the best treatment option based on clinical, angiographic and procedural considerations should also consider that equipoise exists between PCI and CABG in a relevant proportion of subjects. As such, patient preference should also factor in decision-making for borderline cases that are amenable to both procedures.
Acknowledgements
None.
Footnote
Conflicts of Interest: Speaker’s and consulting fees from Abbott Vascular.
References
- Authors/Task Force members, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- Piccolo R, Windecker S, Kolh P. Myocardial revascularization in patients with left main or multivessel coronary artery disease at high surgical risk: conventional wisdom versus risk prediction model. Eur J Cardiothorac Surg 2017;51:949-51. [Crossref] [PubMed]
- Capodanno D, Stone GW, Morice MC, et al. Percutaneous coronary intervention versus coronary artery bypass graft surgery in left main coronary artery disease: a meta-analysis of randomized clinical data. J Am Coll Cardiol 2011;58:1426-32. [Crossref] [PubMed]
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72. [Crossref] [PubMed]
- Park SJ, Kim YH, Park DW, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med 2011;364:1718-27. [Crossref] [PubMed]
- Athappan G, Patvardhan E, Tuzcu ME, et al. Left main coronary artery stenosis: a meta-analysis of drug-eluting stents versus coronary artery bypass grafting. JACC Cardiovasc Interv 2013;6:1219-30. [Crossref] [PubMed]
- Stone GW, Sabik JF, Serruys PW, et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N Engl J Med 2016;375:2223-35. [Crossref] [PubMed]
- Mäkikallio T, Holm NR, Lindsay M, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet 2016;388:2743-52. [Crossref] [PubMed]
- Capodanno D, Bass TA. Revascularization of Unprotected Left Main Coronary Artery Disease: Implications of Evolving Data on Clinical Practice. Circ Cardiovasc Interv 2016;9. [Crossref] [PubMed]
- Capodanno D, Tamburino C. Unraveling the EXCEL: promises and challenges of the next trial of left main percutaneous coronary intervention. Int J Cardiol 2012;156:1-3. [Crossref] [PubMed]
- Khan AR, Golwala H, Tripathi A, et al. Meta-analysis of Percutaneous Coronary Intervention Versus Coronary Artery Bypass Grafting in Left Main Coronary Artery Disease. Am J Cardiol 2017;119:1949-56. [Crossref] [PubMed]
- Capodanno D, Gargiulo G, Buccheri S, et al. Computing Methods for Composite Clinical Endpoints in Unprotected Left Main Coronary Artery Revascularization: A Post Hoc Analysis of the DELTA Registry. JACC Cardiovasc Interv 2016;9:2280-8. [Crossref] [PubMed]
- Capodanno D. Beyond the SYNTAX score--advantages and limitations of other risk assessment systems in left main percutaneous coronary intervention. Circ J 2013;77:1131-8. [Crossref] [PubMed]
- Sotomi Y, Cavalcante R, van Klaveren D, et al. Individual Long-Term Mortality Prediction Following Either Coronary Stenting or Bypass Surgery in Patients With Multivessel and/or Unprotected Left Main Disease: An External Validation of the SYNTAX Score II Model in the 1,480 Patients of the BEST and PRECOMBAT Randomized Controlled Trials. JACC Cardiovasc Interv 2016;9:1564-72. [Crossref] [PubMed]
- Cavalcante R, Sotomi Y, Lee CW, et al. Outcomes After Percutaneous Coronary Intervention or Bypass Surgery in Patients With Unprotected Left Main Disease. J Am Coll Cardiol 2016;68:999-1009. [Crossref] [PubMed]


