Pulmonary metastasectomy for colorectal cancer: analysis of prognostic factors affecting survival
Introduction
In 2012, colorectal cancer (CRC) cases were estimated to be 446.8 thousands in Europe, second only to breast cancer (1). With 214.7 thousands of deaths, CRC was also the second-leading cause of European cancer-related death in both men and women. In Italy in 2012, CRC was the second cancer-related cause of death in males, the second in terms of new cases and death in females and the third in terms of new cases in males (1).
Around 10–20% of patients with CRC developed pulmonary metastases (2). Although pulmonary metastasectomy could be a radical choice for rigorously selected patients (3), a certain quantity of subjects with PM are managed with palliative intent and have a poor outcome. Nevertheless, disappointing results after chemotherapy in patients with advanced CRC, laid solid foundations for pulmonary resection of lung metastases (4,5). In fact, pulmonary metastasectomy is being commonly performed worldwide in selected patients with reported 5-year survival data ranging from 27% to 68% (5-7).
Different prognostic factors including a short disease-free interval between primary tumor resection and lung metastases occurrence, multiple metastatic nodules, and elevated preoperative CEA have been found to be associated with poor survival (7,8). Conversely, the presence of synchronous or metachronous resected liver metastases was not significantly related to poor survival (7).
BRAF is a member of the Ras/Raf/MEK/MAP kinase cascade, which transduces various growth signals from the cell surface to the nucleus. Mutations of the genes encoding KRAS have been implicated in colorectal carcinogenesis (9). BRAF mutations occurred in 5–11% of CRC cases, and BRAF-mutant CRC has been associated with clinicopathological features, including sex, tumor location, and clinical stage (9-11).
Tol et al. reported that patients with BRAF-mutated CRC had a significantly shorter median progression-free and median overall survival (OS) than patients with wild-type-BRAF tumors (12).
Moreover, BRAF mutation status, as well as KRAS, have been shown to be predictive for whether a patient may benefit to EGFR-inhibitors therapy, such as cetuximab (13). Therefore, detection of these mutations is crucial in the clinical scenario of CRC. More and more papers published in the literature, confirm that BRAF mutation detected by immunohistochemistry has a high sensitivity (14). For this reason, we performed an immunohistochemical analysis of BRAF in primary and metastatic tissues to test its prognostic significance.
CDX2 (Caudal-type homeobox transcription factor 2) has been shown to play an important role in the development and differentiation of colonic epithelium; in addition, CDX2 appears to have a tumor suppressor function and it is involved in the carcinogenesis process (15). CDX2 immunohistochemistry is routinely used as a reliable marker in the pathologic examination of pulmonary colorectal adenocarcinoma metastases (15,16). Zhang et al. recently observed that a lack of CDX2 expression in metastatic CRC is associated with worse OS compared with CDX2 positive tumors (16). The objective of this study was to evaluate which factors influenced survival in CRC patients undergoing pulmonary metastasectomy by studying primary tumors and pulmonary metastases.
Methods
All patients undergoing primary CRC resection and subsequent pulmonary metastasectomy during a 10-year period were retrospectively evaluated. Patients’ data were retrieved from hospital medical records and collected in an anonymous electronic database. Follow-up data were acquired by routine surgical outpatient visits or from administrative data (outpatient registry). Follow-up was completed over March 31st 2017. The study was performed according to the Declaration of Helsinki as revised in 2013. Informed consent was waived due to the retrospective nature of the study. At the University Hospital of Parma, at the time of hospital admission, every patient is asked to sign an informed consent on the processing of personal data regarding his/her health state pursuant to Decree-Law No 196 of 30 June 2003 “Italian Personal Data Protection Code”. This document underlined that those data could be used for scientific and statistical research purposes in medical, bio-medical and epidemiological fields.
Eligibility criteria for pulmonary metastasectomy were the followings: primary tumor previously cured or curable; no evidence of extrapulmonary metastases; complete resectability of all pulmonary metastases; planned resection volume tolerable by the patient; no valid therapeutic alternatives available. Preoperative evaluations included clinical examination, blood tests, electrocardiogram, standard chest radiograph, contrast enhanced computed tomography (CT) scan of the chest and abdomen and positron emission tomography (PET-FDG) whole body scan. Spirometry and echocardiogram were performed in case of multiple bilateral pulmonary nodules and previous history of cardiac dysfunction. Arterial blood gas analysis was also always available.
The surgical approach was chosen according to the number and location of the pulmonary nodules. A posterolateral thoracotomy was the access of choice. When feasible and according to surgeon’s attitude, a VATS resection was also performed. In case of synchronous bilateral nodules, patients were operated on sequentially, at distance of about one month. In case of an open approach, a digital palpation of the lung was always performed. Conversely, in case of VATS procedure, an instrumental lung palpation was carried out. Wedge resections were mostly performed; segmentectomies or lobectomies were achieved in the minority of the patients. Lymph-nodes removal was performed only in selected cases (i.e., PET positive).
BRAF CDX2
In order to test the prognostic impact of BRAF and CDX2 in primary CRC and pulmonary metastases, two groups were considered: group A consisted of patients undergoing pulmonary metastasectomy after CRC treatment, including neoadjuvant therapy, surgery or adjuvant therapy; and group B consisted of patients having undergone CRC treatment (including neoadjuvant therapy, surgery or adjuvant therapy) with no history of pulmonary or extra-pulmonary metastases and alive at the time of last follow-up.
Primary tumors were classified according to the TNM AJCC (VII edition) and re-evaluated by three experienced pathologists. In the primary tumor, the presence or absence of the peritumoral inflammatory infiltrate and invasion of lymphatic vessels were evaluated. The tumor was considered mucinous when more than 50% of the tumor tissue was extracellular mucinous. In case of lesions with less than 50% of mucin, an adenocarcinoma with mucinous component was defined.
Immunohistochemistry
Immunohistochemical staining of formalin fixed, paraffin-embedded tissue sections were performed on all samples. After deparaffinization and rehydration, sections were treated with 3% hydrogen peroxidase for 5 mins. For antigen retrieval, sections were treated with pH9 Tris-EDTA buffer for 30 mins in water-bath at 98 °C. Tissue sections were stained with the following primary antibodies: cytokeratin 7 (clone SP52, Roche), cytokeratin 20 (clone SP33, Roche), CDX2 (clone EPR2A64Y, Roche) and BRAF (cloneVE1, Roche). Since more and more papers published in the literature, confirm that BRAF immunohistochemistry showed a high sensitivity (2), BRAF was performed on primary tumors for immunohistochemical analysis. The sections were immunostained with a polymeric system (Ultraview DaB Detection Kit by Ventana-Roche) in accordance with the manufacture’s specifications. Diaminobenzidine (DAB) was used for staining development and the sections were counterstained with hematoxylin. Negative controls consisted of substituting normal serum for the primary antibody.
Outcomes
According to the literature, the following prognostic factors were analyzed: gender, age, tumor location, staging, grading, histological type, vessel invasion, inflammatory infiltrate, neoadjuvant chemotherapy, adjuvant chemotherapy, radiotherapy, chemotherapy, CK7, CK20, extra-pulmonary metastases. OS was calculated from the date of resection to the date of death irrespective from the cause (cancer related or not-related). All alive patients were censored on the date of last follow-up. Survival after pulmonary metastasectomy was also considered; it was calculated from the date of pulmonary resection to the date of death irrespective from the cause. Disease free interval (DFI) was defined as the interval between primary CRC surgery and pulmonary metastasectomy.
Statistical analysis
Qualitative variables were reported as absolute and % frequencies. Quantitative variables were reported as mean (SD) in the absence of deviations from normality (Kolmogorov-Smirnov test). The prognostic power of each single variable was tested by means of univariate models of Cox Regression. In the presence of significant effects, Kaplan-Meier curves followed by log-rank test were performed. Given the relatively low number of subjects, multivariate Cox regression model was performed including those variables that presented a high variation in significance excluding those patients with more metastases and/or maintained a P≤0.20 with and without them. High significant correlation between pairs of predictors was excluded by means of Pearson’s correlation tests. Statistics were performed by using IBM SPSS Statistics v 24 (IBM, Amork, NY, USA) and a P value of 0.05 was considered as significant.
Results
Patient characteristics are shown in Table 1. Fifty-four patients (age 41–86 years, mean 66 years) were evaluated. Fifteen out of 54 patients developed extra-pulmonary localizations beyond pulmonary metastases; thirteen patients had liver metastases, one had adrenal gland metastasis, one patient developed renal and brain metastases beyond lung metastases. Those patients with extra-pulmonary metastases in addition to pulmonary metastases had a significant worse OS than patients with pulmonary metastases alone. Statistical analysis showed that having pulmonary metastases from CRC did not influence OS significantly. Tumor relapse did not rely on these two abovementioned conditions. The removal of pulmonary metastases favorably impacted on patients’ survival. The role of adjuvant therapy was evaluated. Postoperative chemotherapy, radiotherapy or chemo-radiotherapy did not significantly influence the occurrence of pulmonary metastases. Patients having left colon or rectal primary tumor resection, presented significantly higher risk of developing pulmonary metastases with respect to patients with right colon disease. Patients having pulmonary metastases alone had a 10-year survival of 55%. Patients having extra-pulmonary metastases beyond the lung, had a 10-year survival of 0%. The mean follow-up duration was 63 months (5 to 140 months). At the end of follow-up, 23 of 54 patients (43%) had died, and the remaining 31 patients (57%) were alive.
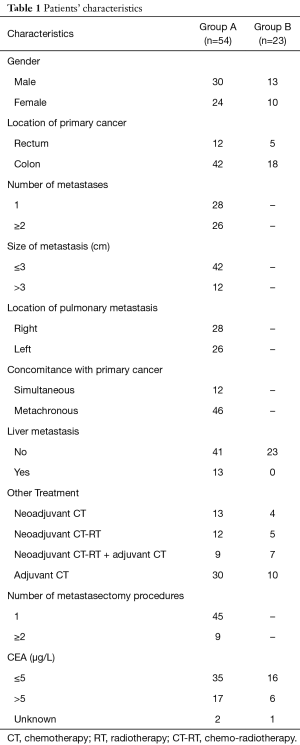
Full table
Immunohistochemistry
Twenty-three patients (age 38–95 years, mean 68) having undergone primary CRC resection who did not develop any pulmonary or extra-pulmonary metastases were considered as control group (group B): eight years after the operation, patients’ survival was 100%. Only one patient was found to be BRAF mutated, the other 22 were negative. Conversely, all patients except one resulted CDX2 positive. Considering group A, immunohistochemistry expression of BRAF did not significantly differ between primary tumor and pulmonary metastases. As expected, BRAF protein expression was located in the cytoplasm, with finely granular cytoplasmic staining. Any nuclear staining was ignored and not scored. No difference with regard to BRAF expression was found between the two groups. In fact, only one single case of BRAF mutation was found in primary CRC and pulmonary metastasis, respectively. Even the expression of CDX2 was evaluated both in primary tumors and metastases. We observed that CDX2 was expressed in 100% of primary CRC. In three cases of pulmonary metastases, we were not able to evaluate the immunohistochemical expression of CDX2, cytokeratin 7 and cytokeratin 20 due to depletion of tissue sample. All the remaining 51 cases of pulmonary metastases were found to be positive for CDX2 expression. Three metastases did show a usual immunophenotypic profile because of the presence of a strong positivity for cytokeratin 7 and CDX2 and a negativity for cytokeratin 20. In these cases, the presence of CDX 2 together with the morphology of the primary tumor and clinical data suggested the diagnosis of CRC metastases. Tumor inflammatory infiltrate did not significantly differ between the two groups.
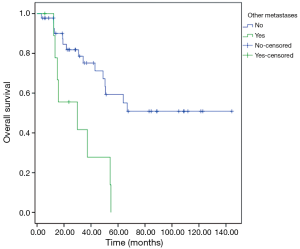
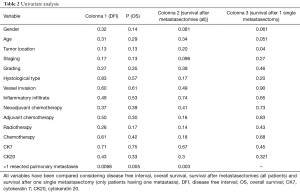
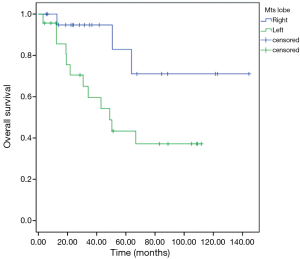
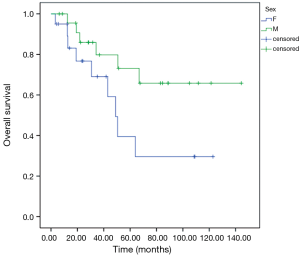
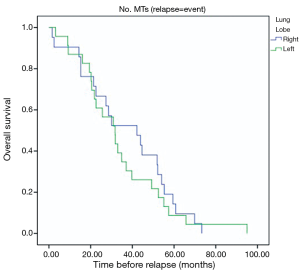
Patients with extra-pulmonary metastases showed a significantly lower survival overall (Figure 1), being the presence of extra-pulmonary metastases the only significant and reliable prognostic predictor of survival (Tables 2,3) considering both OS and survival after pulmonary metastasectomy (Figure 1, P=0.001 with log-rank test vs. P=0.003 with univariate Cox regression). Considering patients undergoing one single pulmonary metastasis resection and survival after metastasectomy, those with right pulmonary metastases presented a significant longer survival than those with left pulmonary metastases (Figure 2, P=0.027 with log-rank test vs. 0.04 with univariate Cox regression). Males had an almost marginally significant longer OS compared to women (Figure 3, P=0.051 with log-rank test vs. 0.061 with univariate Cox regression). DFI was not significantly different in patients undergoing right or left pulmonary metastasectomies (Figure 4). Based on these data and considering p variations from overall model to single metastasis model (Table 2), we constructed a multivariate overall model that included number of resected metastases (relapse P=0.068; survival after metastasectomy P=0.003; OS P=0.005), sex (0.081 vs. 0.061), site of the metastasis (0.20 vs. 0.04), histological type (0.17 vs. 0.20). In this model, males had lower HR than females [0.389 (0.161–0.945, P=0.037)] and left pulmonary metastasis higher HR than right [3.094 (1.161–8.245, P=0.024)]. The presence of multiple metastasis remained the most significant prognostic factor [HR =7.138 (2.433–20.942), P<0.001] (Table 3). The inclusion of other possible not significant predictors did not change relevantly the model (data not shown).
Full table

Full table
Discussion
In our retrospective study evaluating CRC patients who underwent a lung metastasectomy, we observed that the most significant and reliable prognostic factor was the presence of extra-pulmonary metastases. In fact, patients with a history of extra-pulmonary metastases experienced a significant shorter survival compared to patients with pulmonary metastases alone (P=0.001 with log-rank test vs. P=0.003 with univariate Cox regression).
Kim et al. recently published a paper comparing survival between CRC patients with a history of pulmonary and hepatic metastasectomy and those with pulmonary metastasectomy alone (5). They showed that there was no significant difference in the 5-year OS and disease free survival rates between the two groups. These findings are in line with previous studies reported on the outcomes of hepatic and pulmonary metastasectomies. In 2003, Mineo and coauthors, in their series of 29 patients, were unable to show that simultaneous lung and hepatic metastases occurrence was a significant negative prognostic factor (17). A median survival from the second metastasectomy of 41 months, with a 5-year survival rate of 51.3% was reported. For that reason, they concluded that surgery of simultaneous CRC metastases should remain a valid therapeutic option. On the contrary, some authors found that an earlier history of liver metastasectomy was associated with poor survival in patients undergoing pulmonary metastasectomy (18). Landes and coworkers, for example, demonstrated that patients who previously developed liver metastases, have a higher risk of tumor recurrence and a decreased survival in comparison with patients who underwent surgery for pulmonary-only CRC metastases. They argued that their results were clinically relevant, since the incidence of pulmonary metastases without liver metastases is low; so, they deduced that a history of previously resected liver metastases should be considered a poor prognostic factor in CRC patients candidates for pulmonary metastasectomy (18).
We also demonstrated in our study that those patients with right pulmonary metastases presented a significant longer survival than those with left pulmonary metastases (P=0.027 with log-rank test vs. 0.04 with univariate Cox regression). To the best of our knowledge, we could not find any papers in the literature confirming that finding. At the same time, it is hard for any of us to scientifically justify this result. One could speculate that, since the left lung is smaller than the right one and therefore it is less ventilated, a “relative hypoxic condition” could be hypothesized. It is well known that hypoxia within the tumor microenvironment is an important feature of solid tumors. Hypoxia is associated with a scanty prognosis for many cancers, including CRC (19), probably because of hypoxic zones being more resistant to chemotherapy and radiotherapy (20). So primary CRC might be more prone to relapse in the left lung and therefor left metastases could be more aggressive than the those of the contralateral side. Certainly, it is a fanciful assumption, but our finding might be attributed to that mechanism; of course, further studies are warranted to confirm these data in a larger study cohort.
Other findings that could be inferred from our study concerned the mild survival difference we found in males and females. In fact, males had an almost marginally significant longer OS compared to women (P=0.051 with log-rank test vs. 0.061 with univariate Cox regression). Many authors were not able to show any statistical significant difference, in terms of long term survival, between male and female patients after CRC pulmonary metastasectomy (21,22).
Contrariwise, Li et al. found a significant shorter OS in females undergoing pulmonary resection of CRC metastases compared to males (9). In order to evaluate the correlation between BRAF mutation status and negative clinico-pathological features, the authors performed a meta-analysis of 25 studies evaluating more than 13 thousands patients. They observed that patients with BRAF mutation showed 5.8-fold increase in female gender, poor differentiation, higher histological stages, proximal site, and size >5 cm compared with patients with no evidence of BRAF mutation (9). Some former reports revealed that CRC patients with BRAF mutations tend to be at a more favorable clinical stage (23), while other studies indicated that CRC patients with altered BRAF have a poor prognosis (24). With regard to BRAF mutation, we did not observe any statistical difference between primary CRC and pulmonary metastases. In fact, considering group A, only two patients were found to have BRAF mutation, one in the primary CRC and one in the pulmonary metastasis, respectively. Similarly, all patients but one in group B resulted negative for BRAF mutation. Even the expression of CDX2 was homogeneously evaluated both in primary CRC and metastases. We observed that CDX2 was expressed in 100% of primary CRC and 51 out of 54 cases of pulmonary metastases were found to be positive for CDX2 expression as well. Zhang et al. performed a retrospective analysis with the aim to characterize the clinical and pathologic characteristics of CDX2-negative metastatic CRC and to assess the prognostic significance of the lack of CDX2 expression for metastatic CRC (16). They studied 66 patients with CDX2-negative metastatic CRC and 79 patients with CDX2-positive metastatic CRC. They interestingly evidenced that patients with CDX2-negative metastatic CRC were significantly more likely to be female, and to have right-sided primary CRC, poorly differentiated tumors and distant lymph node metastasis. They also underlined that the median progression-free survival after chemotherapy was significantly decreased in patients with CDX2-negative metastatic CRC (16). In our cohort of patients, we could not observe any significant difference related to neoadjuvant or adjuvant treatment strategies. And Finally, in our series, tumor inflammatory infiltrate did not significantly differ between the two groups. Actually, we did not look at the different subsets of lymphocytes that can be found in the tumor microenvironment. The so-called tumor-infiltrating lymphocytes (TILs) have a prognostic value in various primary solid cancer types, including CRC (25,26). Schweiger et al. nicely showed that tumor infiltrating CD8+ and FoxP3 positive cells were associated with disease free survival and OS after pulmonary metastasectomy in a cohort of patients with CRC lung metastases. In fact, CD3+ TILs were found in all resected pulmonary metastatic nodules, highlighting the crucial role of immune system in local tumor microenvironment (26). Our study has several limitations, mostly related to the limited number of patients and the fact that perioperative management of CRC patients was not homogenous in terms of neo-adjuvant or adjuvant treatment. There is certainly the chance for introducing selecting bias in non-randomized studies.
Conclusions
In conclusion, this retrospective study evaluating CRC patients who underwent a lung metastasectomy, showed that the most significant and reliable prognostic factor for survival was the presence of extra-pulmonary metastases. In fact, patients with a history of extra-pulmonary metastases experienced a significant shorter survival compared to patients with pulmonary metastases alone. And instead, BRAF mutation status and CDX2 expression did not seem to have a significant role in this small series of cases. Further studies are warranted to confirm these findings in a larger study cohort.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our Institutional Review Board and was performed according to the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of the study.
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [Crossref] [PubMed]
- Mitry E, Guiu B, Cosconea S, et al. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut 2010;59:1383-8. [Crossref] [PubMed]
- Pastorino U. Lung metastasectomy: why, when, how. Crit Rev Oncol Hematol 1997;26:137-45. [Crossref] [PubMed]
- Sanoff HK, Sargent DJ, Campbell ME, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol 2008;26:5721-7. [Crossref] [PubMed]
- Kim S, Kim HK, Cho JH, et al. Prognostic factors after pulmonary metastasectomy of colorectal cancers: influence of liver metastasis. World J Surg Oncol 2016;14:201. [Crossref] [PubMed]
- Salah S, Watanabe K, Welter S, et al. Colorectal cancer pulmonary oligometastases: pooled analysis and construction of a clinical lung metastasectomy prognostic model. Ann Oncol 2012;23:2649-55. [Crossref] [PubMed]
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. [Crossref] [PubMed]
- Treasure T, Milošević M, Fiorentino F, et al. Pulmonary metastasectomy: what is the practice and where is the evidence for effectiveness? Thorax 2014;69:946-9. [Crossref] [PubMed]
- Li Y, Li W. BRAF mutation is associated with poor clinicopathological outcomes in colorectal cancer: A meta-analysis. Saudi J Gastroenterol 2017;23:144-9. [PubMed]
- Yaeger R, Cercek A, Chou JF, et al. BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer 2014;120:2316-24. [Crossref] [PubMed]
- Vakiani E, Janakiraman M, Shen R, et al. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J Clin Oncol 2012;30:2956-62. [Crossref] [PubMed]
- Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med 2009;361:98-9. [Crossref] [PubMed]
- Kosmidou V, Oikonomou E, Vlassi M, et al. Tumor heterogeneity revealed by KRAS, BRAF, and PIK3CA pyrosequencing: KRAS and PIK3CA intratumor mutation profile differences and their therapeutic implications. Hum Mutat 2014;35:329-40. [Crossref] [PubMed]
- Piton N, Borrini F, Bolognese A, et al. KRAS and BRAF Mutation Detection: Is Immunohistochemistry a Possible Alternative to Molecular Biology in Colorectal Cancer? Gastroenterol Res Pract 2015;2015:753903.
- Barbareschi M, Murer B, Colby TV, et al. CDX-2 homeobox gene expression is a reliable marker of colorectal adenocarcinoma metastases to the lungs. Am J Surg Pathol 2003;27:141-9. [Crossref] [PubMed]
- Zhang BY, Jones JC, Briggler AM, et al. Lack of caudal-type homeobox transcription factor 2 expression as a prognostic biomarker in metastatic colorectal cancer. Clin Colorectal Cancer 2017;16:124-8. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Tonini G, et al. Longterm results after resection of simultaneous and sequential lung and liver metastases from colorectal carcinoma. J Am Coll Surg 2003;197:386-91. [Crossref] [PubMed]
- Landes U, Robert J, Perneger T, et al. Predicting survival after pulmonary metastasectomy for colorectal cancer: previous liver metastases matter. BMC Surg 2010;10:17. [Crossref] [PubMed]
- Yoshimura H, Dhar DK, Kohno H, et al. Prognostic impact of hypoxia-inducible factors 1alpha and 2alpha in colorectal cancer patients: correlation with tumor angiogenesis and cyclooxygenase-2 expression. Clin Cancer Res 2004;10:8554-60. [Crossref] [PubMed]
- Nijhuis A, Thompson H, Adam J, et al. Remodelling of microRNAs in colorectal cancer by hypoxia alters metabolism profiles and 5-fluorouracil resistance. Hum Mol Genet 2017;26:1552-64. [Crossref] [PubMed]
- Guerrera F, Mossetti C, Ceccarelli M, et al. Surgery of colorectal cancer lung metastases: analysis of survival, recurrence and re-surgery. J Thorac Dis 2016;8:1764-71. [Crossref] [PubMed]
- Lang C, Hrdliczka E, Schweiger T, et al. Impact of cyclooxygenase-2 and prostaglandin-E2 expression on clinical outcome after pulmonary metastasectomy. J Thorac Dis 2017;9:621-35. [Crossref] [PubMed]
- Yuen ST, Davies H, Chan TL, et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res 2002;62:6451-5. [PubMed]
- Xu Q, Xu AT, Zhu MM, et al. Predictive and prognostic roles of BRAF mutation in patients with metastatic colorectal cancer treated with anti-epidermal growth factor receptor monoclonal antibodies: a meta-analysis. J Dig Dis 2013;14:409-16. [Crossref] [PubMed]
- Anitei MG, Zeitoun G, Mlecnik B, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res 2014;20:1891-9. [Crossref] [PubMed]
- Schweiger T, Berghoff AS, Glogner C, et al. Tumor-infiltrating lymphocyte subsets and tertiary lymphoid structures in pulmonary metastases from colorectal cancer. Clin Exp Metastasis 2016;33:727-39. [Crossref] [PubMed]


