Extracorporeal membrane oxygenation system as a bridge to reparative surgery in ventricular septal defect complicating acute inferoposterior myocardial infarction
Introduction
Postinfarction ventricular septal defect (VSD) is a rare but potentially lethal complication of acute myocardial infarction. Nowadays, the incidence of this complication has fallen to 0.17–0.31% because of early percutaneous revascularization therapy. Nevertheless, the mortality of postinfarction VSD has remained stable (1), 94% with medical management and 43% with surgical treatment (2).
Probably the most important factor for patient survival after postinfarction VSD is the timing of surgery. So, patients whose clinical status forces to surgery within first week postmyocardial infarction has 54% of mortality this rate dropped to 18% if clinical status allowed for delay greater than first week (1,2).
Therefore patients in cardiogenic shock could benefit from preoperative mechanical circulatory support as an option to allow clinical stabilization to delay the surgery allowing better surgical results and greater survival.
In this article, we describe a successfully case of extracorporeal membrane oxygenation system (ECMO) as a bridge to delay reparative surgery in VSD complicating acute myocardial inferoposterior infarction.
Case presentation
A 55-year-old man with no previous medical history, he attended the emergency-room for 12-hour evolution of oppressive chest pain and strong anginal pain 7 days before.
On physical examination, the blood pressure was 96/70 mmHg, the heart rate was 110 bpm, yugular ingurgitation and pansystolic III/VI murmur was noted at the left-sternal border without pulmonary crackles, nor edemas in lower extremities.
An electrocardiogram revealed sinus tachycardia at 110 bpm, 2 mm elevation ST and Q waves in inferior-posterior leads (Figure 1A).
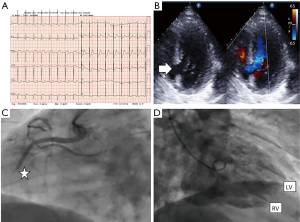
A Transthoracic echocardiogram performed in the emergency room showed undilated left ventricle (LV) with inferior-posterior akinesia, large and complex posterior-basal septal rupture (diameters of 20 mm × 21 mm) with left-right shunt (Figure 1B). LVEF was 50%, without left ventricular free wall rupture, nor pericardial effusion. Right ventricle with normal size and function.
Suspecting VSD in evolved inferoposterior acute myocardial infarction, we performed emergency coronarography using the femoral artery approach. This showed complete subacute occlusion of the mid segment of the right coronary artery (Figure 1C), significative stenosis in mid segment of the left descendent anterior artery and significant diffuse stenosis in left circumflex artery. Left ventriculography showed an undilated left ventricle (LV), with inferior-posterior akinesia and passage of contrast to the right ventricle (Figure 1D). Postventriculography, an intra-aortic balloon pump (IABP) was implanted.
On arrival at the cardiac intensive care unit, the patient was without thoracic pain or dyspnea due to morphine i.v. administered in the emergency room. His blood pressure was 97/60 with IABP, heart rate 95 bpm, oxygen saturation 98% with nasal cannula of low-flow oxygen-therapy at 2 liters-min. He had adequate peripheral perfusion, no yugular ingurgitation, and without signs of pulmonary congestion. Swann-Gantz system was placed through right yugular cannulation, showing central venous pressure of 10 mmHg, capilar pulmonary pressure 12 mmHg and pulmonary wedge pressure 38 mmHg. So we infused volume and low doses of diuretics i.v., along with simple anti-aggregation and oxygen therapy.
The patient was assessed by the Heart Failure-Cardiac Transplant Unit rejected for cardiac transplantation due to his age. Considering the high surgical risk to early surgery and his hemodynamic and clinical stability without inotropes, with parameters of renal function, lactate, pH, and diuresis preserved in the first hours; we decided medical treatment and stabilization until a delay surgical repair treatment (7–10 days later).
But 4 days after admission, the patient suffered hemodynamic instability so venoarterial ECMO was implanted as a bridge to reparative surgery with excellent outcome. On the 9th day after admission (5 days post-ECMO surgery) at which time double bypass with left internal mammary artery graft to the LAD and saphenous vein graft to the Circumflex, VSD repair with a pericardial two-patch exclusion technique, and ECMO decannulation were performed.
The patient’s postoperative course was free of complications, IABP was removed on 3rd postoperative day and was discharged 10 days post VSD repair surgery. Transthoracic echocardiography performed prior to discharge showed an intact VSD repair, and mid-range left ventricular ejection fraction (Figure 2A,B).
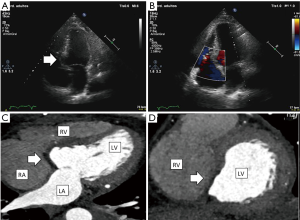
Follow-up 3-month later revealed the patient to be in good functional status. Cardiac computed tomography (CT) was performed showing undilated left ventricle (LV), with inferior and posterior akinesia, with intact interventricular septal patch without shunt (Figure 2C,D).
Discussion
VSD treatment is very complex, because medical management is usually futile, so definitive surgery remains the treatment of choice (3) but the risk surgery is very high and the optimal timing for surgery is still under debate. For all this, the management of this complication is a real challenge (1-3).
Medical management of VSD aims afterload reduction to increase effective LV stroke volume by reducing left-to-right shunting. Intravenous afterload-reducing pharmacotherapy is faster than oral agents. IABP provides mechanical afterload reduction and increased diastolic perfusion of coronary arteries, and may be considered routine care, even in patients who remain hemodynamically stable, as development of haemodynamic compromise is often unexpected, rapid, and fatal (3).
When IABP and medical management fail to stabilize patients in cardiogenic shock, the ECMO should be considered as an option to stabilize these patients and thus allow time to transfer, delay and development of a surgical plan for VSD repair (1).
Peripherally placed Mechanical Circulatory Support (MCS) support devices as ECMO, are less invasive and more accessible than centrally cannulated devices. This is especially important in the setting of acutely evolving myocardial infarctions that may require immediate support (1).
Joint use of ECMO and IABP helps to reduce the left ventricular intention associated with inadequate decompression and decreased myocardial oxygen requirements (1,4).
Several case studies in the literature have documented the use of ECMO to stabilize patients until surgery can be performed. Both as a bridge to recovery (1), and as a bridge to transplantation (4) as a bridge to delayed reparative surgery (1).
Our case, although with the limitations of a single observation, is an interesting case that allows us to remember the importance of an integral management of the patient with postinfarction mechanical complication. An adequate delayed surgical repair strategy allowed a successful surgical outcome with lower mortality. The ECMO were necessary as a bridge to the surgical procedure to stabilize our patient in cardiogenic shock despite the IABP and medical treatment.
It is necessary to emphasize the importance of early diagnosis and the early implantation of MCS with ECMO to avoid multi-organ failure and thus allow a successful outcome in an often fatal complication following myocardial infarction.
Conclusions
ECMO as a bridge to reparative surgery in postinfarction VSD is an adequate option to stabilize patients until surgery, when the cardiac function can be recovered with either primary repair or transplant, medical treatment to stabilization are unsuccessful, and emergent surgery has prohibitive risk.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Hobbs R, Korutla V, Suzuki Y, et al. Mechanical circulatory support as a bridge to definitive surgical repair after post-myocardial infarct ventricular septal defect. J Card Surg 2015;30:535-40. [Crossref] [PubMed]
- Arnaoutakis GJ, Zhao Y, George TJ, et al. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg 2012;94:436-43; discussion 443-4. [Crossref] [PubMed]
- Jones BM, Kapadia SR, Smedira NG, et al. Ventricular septal rupture complicating acute myocardial infarction: a contemporary review. Eur Heart J 2014;35:2060-8. [Crossref] [PubMed]
- Pascual I, López F, Hernández-Vaquero D, et al. Circulatory Support With Extracorporeal Membrane Oxygenation System as a Bridge to Heart Transplantation in Complex Postinfarction Ventricular Septal Rupture. Rev Esp Cardiol (Engl Ed) 2016;69:617-9. [Crossref] [PubMed]


