Recipient selection process and listing for lung transplantation
General indications for lung transplantation
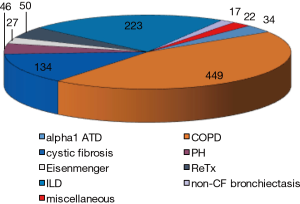
Lung transplantation is performed with increasing numbers all over the world and in the most recent official lung and heart-lung transplant registry report released by the International Society for Heart and lung Transplantation (1), it was mentioned that in 2014 some 4,000 lung transplantation have been performed worldwide, of which 75% were double lung transplants. In this report, which accumulated over 50,000 lung transplantations from 1995 till June 2015, COPD with and without alpha1 antitrypsin deficiency accounted for 36.5%, interstitial lung disease [including idiopathic pulmonary fibrosis (IPF)] for 29.7%, cystic fibrosis (CF) for 15.8%, non-CF bronchiectasis for 2.7%, pulmonary hypertension for 4.4%, retransplantation for 4.1%, and some less common indications such as sarcoidosis, lymphangioleiomyomatosis (LAM), obliterative bronchiolitis, etc. for 6.8% of the total number. These data are gathered from 134 collaborating centers all over the world. In our own center in Leuven (Belgium), the underlying diseases for lung transplantation are shown in Figure 1, and are quite representative for the registry data.
From the beginning of our centers activity, we have used the available guidelines for the selection of lung transplant candidates, although the first international guidelines were only published in 1998 by Maurer et al. This was a joint guidelines paper, produced by ATS, ISHLT, AST and ERS and simultaneously published in the Journal of Heart and Lung Transplantation, Heart Lung and Transplantation (2-4). This paper focused on general medical conditions which impact on eligibility for lung transplantation and clearly indicated an age limit of up to 55 years for heart-lung, 65 years for single lung and 60 years for double lung transplantation. Also disease specific criteria were already mentioned.
In the next guidelines paper, published in the Journal of Heart and Lung Transplantation in 2006, by Orens et al. (5), it was clearly stated that evolving technology and advances in medical knowledge mandated a need for an update. In this revision, age >65 years was only considered as a relative contra indication, given the enhanced experience with such patients. The paper also made a distinction between referral guidelines and transplantation guidelines, which was quite elegant to use at the time. There were no new criteria for pediatric transplantation nor for retransplantation.
The most recent update of the guidelines was published in 2014, by Weil et al. and will form the further basis for this chapter (6).
As the mortality rate after lung transplantation relative to other solid-organ transplants is high and the availability of donor lungs remains limited, lung transplantation should be offered to those in whom a survival benefit can be expected. Overall median survival in most recent reports is 5.8 years with an unadjusted survival rate at 5 years of 54% (1). However, the median survival rate according to the underlying pulmonary disease is very different, varying from 2.8 years after retransplantation to 8.9 years for CF.
Thus, selected adult patients should have chronic, end-stage lung diseases and meet the following criteria:
- High risk of death (>50%) within 2 years if lung transplantation is not performed;
- High likelihood (>80%) of surviving at least 90 days after lung transplantation;
- High likelihood (>80%) of 5-year post-transplant survival;
- No other treatment option possible/available.
Contraindications
The ISHLT’s 2014 guidelines include absolute and relative contraindications. These are of course to be interpreted with some caution, as experienced centers may have other contra indications compared to starting centers. What is really to be considered is the fact that in the below mentioned conditions, there should at least be in depth discussion with the transplant team and the patient, whether a lung transplantation is indeed the right option for this particular patient. Some of these absolute contraindications may also be temporary as for instance a patient may lose weight and decrease a BMI to <35 kg/m2, or an active Mycobacterium tuberculosis infection may be treated for several months before reconsidering the patient for lung transplantation.
Absolute contraindications to lung transplantation
- Recent history of malignancy. A 2-year disease-free interval and a low predicted risk of recurrence may be acceptable, for instance, in localized squamous or basal cell skin cancer, appropriately treated. However, a 5-year disease-free interval is required in most cases, particularly for patients with a history of hematologic malignancy, sarcoma, melanoma, or cancers of the breast, bladder, or kidney. For patients with a history of bronchial carcinoma, for instance, the risk of recurrence may remain too high. A specific condition may be localized prostate cancer, even diagnosed at the time of pre transplant work up, with a Gleason score of max. 3+3 may be acceptable in some patients, although data remain scarce.
- Untreatable significant dysfunction of another major organ system (e.g., heart, liver, kidney, or brain) unless combined organ transplantation can be performed. Several combined organ transplantations have been performed worldwide, with variable outcome, again depending on the experience of the center. Typical examples are combined liver-lung, lung-kidney, heart-lung and liver, lung, kidney, pancreas. Survival with lung-liver in CF patients is reported to be comparable to transplantation of lungs only (7,8).
- Uncorrected atherosclerotic disease with suspected or confirmed end-organ ischemia or dysfunction and/or coronary artery disease not amenable to revascularization.
- Acute medical instability, including, but not limited to, acute sepsis, myocardial infarction, and liver failure. In our own center one patient was transplanted with end-stage COPD and drug-induced acute liver failure. She is doing well >3 years after the procedure (9). This illustrates that such combined transplantations may be feasible, but should be very well discussed before to proceed.
- Uncorrectable bleeding diathesis.
- Chronic infection with highly virulent and/or resistant microbes that are poorly controlled pre-transplant. Human immunodeficiency virus (HIV) and hepatitis B or C are no longer considered as absolute contra-indications, provided patients are treated for HIV and there is no viremia for hepatitis B and C (10,11).
- Evidence of active Mycobacterium tuberculosis infection.
- Significant chest wall or spinal deformity expected to cause severe restriction after transplantation. Morbus Bechterew may be one of these specific conditions. If indeed the mobility of the thoracic cage is severely restricted pretransplant, many problems may arise after transplantation, such as difficult weaning and restrictive pulmonary function with ongoing dysfunctionality.
- Class II or III obesity [body mass index (BMI) ≥35.0 kg/m2].
- Current non-adherence to medical therapy or a history of repeated or prolonged episodes of non-adherence to medical therapy that are perceived to increase the risk of non-adherence after transplantation. This is sometimes difficult to assess, but is very important as it was recently shown that adherence after transplantation may also be problematic, although this was less problematic after lung transplantation (12).
- Psychiatric or psychologic conditions associated with the inability to cooperate with the medical/allied health care team and/or adhere with complex medical therapy.
- Absence of an adequate or reliable social support system.
- Severely limited functional status with poor rehabilitation potential.
- Substance abuse or dependence (e.g., alcohol, tobacco, marijuana, or other illicit substances). In many cases, convincing evidence of risk reduction behaviors, such as meaningful and/or long-term participation in therapy for substance abuse and/or dependence, should be required before offering lung transplantation. Serial blood and urine testing can be used to verify abstinence from substances that are of concern. Prior tobacco smoking is common in patients who have end-stage lung disease, especially COPD and IPF. It is generally assumed that an abstinence period of 6 months might be sufficient before transplantation (or listing), although we recently demonstrated that the time of smoking cessation before transplantation inversely correlated with resumption of smoking after lung transplantation. Moreover, other smoking family members in the household of the patient are another risk factor to resume smoking afterwards. Therefore, presently we also try to convince everyone living in the same house to quit smoking (13).
Relative contraindications
Relative contraindications remain a matter of debate, indeed what is relative in one center may be absolute in another center. This greatly depends on the experience of the center. All these relative contraindications should therefore be interpreted with caution, and too many relative contraindications may become an absolute contra indication for lung transplantation.
- Age >65 years in association with low physiologic reserve and/or other relative contraindications. It is evident from the literature that older patients have a worse outcome. This was clearly identified in the ISHLT registry report from 2013 (14) which focused on age. Between 2003 and 2012, 19,930 lung transplants were registered, of which 10% were in patients >65 years of age. Survival not only varies by era, and by underlying disease, within each diagnostic group, older patients had a worse survival and in general the 5-year survival in >65 years was 38%, compared to 46% for those between 60–65 years and 52% to 57% for those <60 years (14). Of course, age is not the only factor that counts, also frailty of the patient is important. A frail patient <60 years may have a worse prognosis compared to a non-frail >65 years old. All factors should indeed be taken into account when considering an older patient for lung transplantation. Whether older patients may benefit more from a single than from a double lung remains a matter of debate, as there are a lot of contradictory studies.
- Class I obesity (BMI: 30.0–34.9 kg/m2), particularly truncal (central) obesity and progressive or severe malnutrition, with a BMI <14. Although there is evidence from large databases that as well overweight as underweight patients suffer from a worse survival compared to normal weight patients (15,16), the debate is still going on. We have recently investigated the role of BMI on outcome after lung transplantation in 546 LT recipients, of which 28% had BMI <18.5 kg/m2. Underweight resulted in similar survival (P=0.28) compared to the normal weight group. Significantly higher mortality was found in overweight (P=0.016) and obese patients (P=0.031) compared with the normal-weight group. Subanalysis of either underweight (P=0.19) or obese COPD patients (P=0.50) did not reveal worse survival. In patients with interstitial lung disease, obesity was associated with increased mortality (P=0.031) compared to the normal-weight group. In CF patients, underweight was not associated with a higher mortality rate (P=0.12) compared to the normal-weight group (17).
- Severe, symptomatic osteoporosis. This might indeed result in further vertebral fractures that may compromise breathing, coughing and rehabilitation after lung transplantation, leading to a more complicated postoperative course.
- Extensive prior chest surgery with lung resection. This greatly depends on the experience of the surgeons and the center. In a rather old series investigating this subject, it was found that in carefully selected cases, previous thoracic surgery had no major impact on lung transplantation outcome. The perioperative risk and the transfusion requirements were not elevated compared to patients without previous thoracic surgery. However, the surgical procedure itself was more difficult (18).
- Mechanical ventilation and/or extracorporeal life support (ECLS). Nowadays, there is increasing experience with extra corporeal membrane oxygenation (ECMO) as a bridge to lung transplantation; In experienced centers, this has little impact on survival, however, patients need to be carefully evaluated and the center volume seems very important and does impact on survival (19). Recently, there is emerging evidence that awakes ECMO which enables further rehabilitation, may lead to improved results after lung transplantation, with a 2-year survival of 81% (20).
- Colonization or infection with highly resistant or highly virulent bacteria, fungi, and certain strains of mycobacteria (e.g., chronic extrapulmonary infection expected to worsen after transplantation). This again is highly dependent on the experience of the center. A classic example is the presence of Mycobacterium abscessus in CF patients, which is in some centers an absolute contraindication, whereas in others it is a relative one (21,22). Patients who are infected with Burkholderia cenocepacia or Burkholderia gladioli are a particular challenge for lung transplantation and could be considered for transplantation if the infection is sufficiently treated preoperatively and if there is a reasonable expectation for adequate control postoperatively (23,24). For patients infected with hepatitis B and/or C, a lung transplant can be considered when there are no significant clinical, radiologic, or biochemical signs of cirrhosis or portal hypertension; moreover, the patients should be stable on appropriate therapy. For patients infected with HIV, a lung transplant can be considered in those with controlled disease with undetectable HIV-RNA, and compliant on combined anti-retroviral therapy (25). In general, patients with these infections should be evaluated by a transplant center with significant experience managing these infections, and patients should be informed of the increased risk of transplantation.
- Atherosclerotic disease burden without end-organ disease. With regard to coronary artery disease, some patients will be candidates for percutaneous coronary intervention or simultaneous coronary artery bypass graft (CABG). The preoperative evaluation, type of coronary stent used (bare metal vs. drug eluting), and degree of coronary artery disease that is accepted vary among transplant centers. In our own experience, we treated 23 patients out of a total of 775 isolated lung transplantation procedures with either one or two stents (n=20) or simultaneous CABG (n=3). The survival rates were similar in both groups, illustrating that preoperative or intraoperative correction of the coronary stenosis results in a similar outcome as in patients with no coronary stenosis.
- Other medical conditions that have not resulted in end-stage organ damage, such as diabetes mellitus, systemic arterial hypertension, epilepsy, central venous obstruction, peptic ulcer disease, or gastroesophageal reflux, should be optimally treated before transplantation. Gastroesophageal reflux is highly prevalent in lung transplantation candidates and is often asymptomatic, requiring invasive testing for diagnosis (26); moreover, in general, GERD worsens after transplantation (27). GERD is accepted to be a risk factor for allograft dysfunction after lung transplantation, especially acute rejection (28) and bronchiolitis obliterans syndrome (BOS) (29,30). Treatment may require surgical intervention, such as a Nissen fundoplication. Whether a possible treatment should be performed before or after transplantation, especially in the prevention of BOS, remains a matter of debate. Indeed preventive surgery may lead to a better postoperative FEV1, but does not necessarily impact on the occurrence of BOS (31-34). A special consideration is the patients with scleroderma and esophageal dysfunction/motility with reflux. Although this condition was initially regarded as a contra indication for lung transplantation, at least in some centers, recent evidence suggests that in selected patients, even those with gross reflux and esophageal dysfunction, results are acceptable, with a 5-year survival of 70%. Also, the prevalence of BOS was comparable to non-scleroderma patients (35).
Specific disease related referral and transplantation criteria
Because of the existence of a transplantation window, this means the time between activation on the waiting list and the transplantation procedure, which varies according to the underlying condition and per transplant center, it is obvious that patients need to be referred in time. As a consequence, a differentiation has been made between referral criteria for lung transplantation (meaning at the start of the transplant window) and transplantation criteria. This difference between referral and transplantation criteria was first used in the 2006 guidelines (6), and again in the revised 2014 guidelines (7). This is of utmost importance, since, in general, the waiting time varies according to the underlying disease which is an important factor to calculate the lung allocation score (LAS). Indeed the LAS, which means the urgency of a transplantation procedure, is much higher for a patient with for instance IPF compared to a stable COPD patient, illustrating that a patient with IPF will have a shorter waiting time. This all needs to be taken into account when referring a patient to a local transplant center (36).
Chronic obstructive pulmonary disease and alpha1-antitrypsin deficiency
COPD remains the most prevalent indication for lung transplantation, with 36.5% of all procedures being performed worldwide between Jan 1995 and Jun 2015. Of these, 57.3% are double lung transplantations (1). Although COPD should constitute a simple diagnosis, there is much heterogeneity which makes it often difficult to adhere to strict transplantation criteria. This is very well illustrated by the recent 2017 GOLD guidelines, which not only stage COPD based on FEV1 but also on dyspnea, number of (severe) exacerbations and co morbidities.
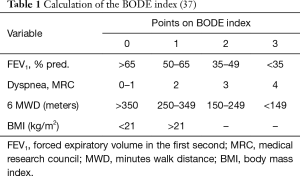
In general, prognosis of COPD depends on the severity of the airway obstruction, breathlessness, number of exacerbations and functional limitation. This is reflected in the BODE index, which points to survival rates (Table 1) (37). Although the BODE index may be used as prognosticator for COPD patients in general, there is debate whether this can also be used in selected COPD patients who may qualify for lung transplantation (38). Indeed, in this latter population, there is in general less comorbidity, which may impact on survival. Nevertheless, BODE index has been used in COPD patients who qualify for lung transplantation and seems to be a help to identify suitable transplant candidates with COPD (39,40). A BODE score of 7–10 was associated with a mortality of 80% at 4 years, whereas a score of 5–6 conferred a mortality of 60% at 4 years, and proved to be a better indicator of survival than the spirometric staging system. Indeed, Lahzami et al. evaluated the role of the BODE score in lung transplantation for COPD and demonstrated that most patients with COPD had an individual survival benefit from lung transplantation regardless of their pre-transplant BODE score, although a global survival benefit was only seen in patients with a BODE score ≥7, suggesting that this is the appropriate population to transplant (41).
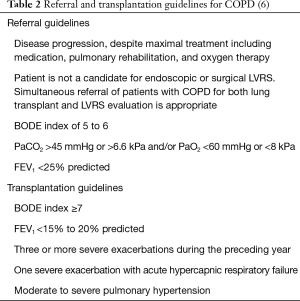
Exacerbation frequency and the severity of exacerbations is also a known prognosticator in COPD; indeed, the presence of ≥3 exacerbations/year negatively affects survival in patients with COPD (42). The increased mortality risk is independent of the BODE index (43). Acute hypercapnic respiratory failure increases the in-hospital mortality to 41%, and to a further 43% and 49% after 1 and 2 years in survivors (44). In another cohort, the 1-year mortality in patients who needed non-invasive ventilation during an acute COPD exacerbation was 30% (45). Specific referral and transplantation guidelines for patients with COPD are summarized in Table 2.
As already stated in the general indications for lung transplantation, there should no other treatment option be available, besides lung transplantation. This off course includes rehabilitation and also the possibility of lung volume reduction (LVR), either endoscopically (E) or surgically (S). According to the NETT trial, patients with an FEV1 of less than 20%, as well as a diffusing capacity of the lungs for carbon monoxide (D
One-way endobronchial valve (EBV) placement is the best studied approach and is targeted to the most emphysematous destroyed lung lobe. The first trials showed some benefits on pulmonary function parameters but the results were considered as not clinically meaningful. However, post-hoc analyses demonstrated that patients with an intact interlobular fissure on HRCT scan experienced the best outcome following EBV implantation (47). The STELVIO study compared EBV treatment (n=34) versus standard care (n=34) in a selected group of patients with severe emphysema and absence of collateral ventilation. In the intention-to-treat population, a statistical and clinical significant improvement of FEV1 (∆140 mL or ∆17.8%), forced vital capacity (FVC) (∆347 mL or ∆14.4%) and 6-minute walking distance (6 MWD) (∆74 m or ∆23.3%) was observed after 6 months. When focusing on the per-protocol analysis, [intervention (n=25) vs. control (n=33)], the effect was even larger [+191 mL for FEV1 (95% CI, 109–272 mL), + 442 mL for FVC (95% CI, 215–668 mL)] and +106 m on 6 MWT (95% CI, 80–133 m) and also resulted in major changes in quality of life (−14.9 difference on SGRQ). A similar magnitude of effects was observed in the standard treatment group that switched to the intervention after 6 months (46). Moreover, data on patients in further follow-up (n=40/64) confirmed sustained benefits with 65%, 63% and 75% responders after 1 year taking into account the minimal clinical important differences (MCID) for respectively FEV1, SGRQ and 6 MWT (48).
In that respect, LVRS or LVRE might deviate some selected COPD patients from lung transplantation, or on the other hand may temporarily improve these patients which may result in better rehabilitation potential and in a better outcome after a subsequent lung transplantation.
Diffuse parenchymal lung disease (DPLD)
DPLD constitutes about 30–35% of all indications for lung transplantation, with IPF being the largest indication amongst all DPLD (1). The other DPLD for which transplants are also performed include LAM, histiocytosis X, sarcoidosis, and collagen vascular disease-associated interstitial lung diseases, such as scleroderma, polymyositis and rheumatoid arthritis.
IPF and fibrotic non-specific interstitial pneumonia (NSIP)
Prognostic factors
Over the last years, a great interest has been developed in IPF, since the introduction of antifibrotic drugs, which was the first ever treatment for this disease that seemed to slow its progression and to impact on mortality. Several guidelines for diagnosis of IPF have been released, mainly based on radiologic criteria, with an UIP pattern being associated with a worse prognosis (49,50). It became clear that the prognosis of IPF is indeed very bad, with a 50% survival of 2–3 years after the diagnosis (51,52), which is much worse compared to other DPLD. Several disease characteristics that have an impact on prognosis have been identified such as the % pred., FVC at diagnosis, the rate of decline of FVC over 6 months, the diffusing capacity for CO and the occurrence of exacerbations (53).
Indeed, Nathan et al. showed that patients divided by FVC (mild, ≥70%; moderate, 55% to 69%; and severe, <55%) had correspondingly worse median survival of 55.6, 38.7, and 27.4 months, respectively (54). Also, longitudinal change in FVC has been demonstrated to be a risk factor for increased mortality in multiple studies, and even marginal changes in FVC (5% to 10%) over a 6-month were associated with a higher mortality than in patients with stable disease (53,55). Furthermore, also D
As a consequence, timely referral of these patients is very much needed. This is even more of interest as an important survival benefit after transplantation for IPF has been demonstrated in several series (53,61). Criteria for referral and listing are summarized in Table 3.
For other DPLD (for instance LAM, ILD associated with collagen vascular diseases), no clear criteria have been issued so far, but if the pulmonary disease is severe enough to warrant consideration of lung transplantation and the lung disease has not responded to appropriate treatment and there are no extrapulmonary contraindications to transplantation, it is reasonable to use similar guidelines to those proposed for IPF (7). For sarcoidosis, patients can be referred for transplantation if they are at least in New York Heart Association class III, and should be transplanted if they meet one of these further criteria:
- Hypoxemia at rest;
- Pulmonary hypertension;
- Elevated right atrial pressure >15 mmHg.
A specific caution for IPF patients on antifibrotic treatment (pirfenidone of nintedanib) needs to be mentioned: despite these drugs have an impact on disease progression, on exacerbation rates and on survival (62-65), this treatment may not delay referral of IPF patients for consideration of lung transplantation, but it may buy time on the waiting list as in some countries wait list mortality for IPF is still very high, up to >30% (66). On the other hand, treatment with these drugs has no adverse outcome after transplantation and does not need to be stopped when patients are listed (67).
CF and non-CF bronchiectasis
CF is one of the major indications for lung transplantation; accounting for about 15% of all transplants in 2014 (1). CF mostly affects younger patients compared to other diagnosis necessitating lung transplantation. The survival is therefore reported to be consistently higher, with a mean 5-year survival of 62.5%, compared to 53.8% for COPD and 48.5% for idiopathic interstitial diseases (1). In our own experience, the actuarial 5- and 10-year survival in a cohort of CF patients transplanted between 2005 and 2015 (n=81) was 90% and 86% respectively (68).
Non-CF bronchiectasis may have different causes, and in a recent study, among the 1,258 patients enrolled, an etiology of bronchiectasis was determined in 60%, including post-infective (20%), chronic obstructive pulmonary disease related (15%), connective tissue disease related (10%), immunodeficiency related (5.8%), and asthma related (3.3%). In 40% of patients, there was no specific cause identified (idiopathic bronchiectasis) (68). Although mostly older than CF patients (mean age 67 years, 58–75 years) (69), the referral and transplantation criteria are comparable to CF patients (Table 4).
These referral and transplantation criteria are mainly based on the publication by Kerem et al. (70). These authors analyzed mortality predictors in 673 CF patients, and clearly demonstrated that the prognosis of CF patients is related to FEV1 (<30% pred.), a PaO2 <55 mmHg, a PaCO2 >50 mmHg and the BMI. They also concluded that female patients and younger patients had a worse prognosis (70). Since the publication of Kerem et al., prognosis of CF patients has surely improved, but nevertheless, these criteria are still useable when evaluating a CF patient for possible transplantation.
Several predictive models for 5-year survival have been published so far, and although mostly valid in the tested population, they proved to be wrong in a control population. This may have to do with local treatment options and habits.
Special considerations in CF patients
Microbial and fungal colonization of the airways is abundant in CF patients. As already mentioned in the general contra-indications section of this chapter, colonization with B. Cepacia and especially Burkholderia cepacia complex (BCC) or Burkholderia cenocepacia may be a contra-indication for transplantation in some centers. Indeed, the Newcastle group recently published their experience with lung transplantation in CF patients with BCC: of 216 CF patients transplanted, 22 had BCC of whom 12 Burkholderia cenocepacia. Nine Burkholderia cenocepacia-infected recipients died within the first year, and 8 sepsis were considered to be the cause of death. These results lead this group to further decline patients with pre-transplant colonization with Burkholderia cenocepacia (71), whereas this may be an acceptable risk for others. Acceptance of such patients for lung transplantation will thus depend on experience of the team and initial outcomes with such patients.
On the other hand, CF patients colonized with Achromobacter xylosoxidans and Stenotrophomonas maltophilia have similar post-transplant survival as compared to other CF patients, irrespective of their antibiotic susceptibility patterns. The presence of these organisms should not preclude lung transplantation (72).
Colonization of the airways with Mycobacterium abscessus also leads to conflicting results, with some centers having good outcome whereas others do regard this colonization as a contra-indication (see section on general contra-indications) (21,22).
Colonization with Scedosporium apiospermum may have an identical impact, with some centers declining such patients and others accepting them, provided they are actively treated with azole derivatives and receive lifelong azole treatment after transplantation when they get colonized (73).
Some CF patients have overt liver disease, evolving to cirrhosis, which is recognized as an independent risk factor for death or lung transplantation. In that case, a combined liver and lung transplantation procedure can be performed, with good outcome. We recently reported our own experience with 11 combined lung and liver transplantations, of which five patients had CF. The 5-year patient survival was 90% (8).
Pulmonary arterial hypertension (PAH)
PAH, and more specifically idiopathic PAH (iPAH) and chronic thromboembolic PAH remain a valid indication for lung transplantation (1,6). The number of lung transplantations for these conditions has gradually decreased, given the better treatment options that have become available over the last 10 years. Nowadays, <3% of all indications for lung transplantation are performed in iPAH and <2% in non-iPAH patients (1). If patients with PAH fail their usual (triple) treatment regimen (prostanoids, endothelin receptor antagonists, and phosphodiesterase inhibitors), they might need a lung transplant. For most of these patients who can become very debilitated in a short time, the transplant window is often very short, and sometimes, they will need a rather urgent transplant. In that case, the LAS may help to prioritize patients with PAH for transplantation, as has recently been shown in Germany. Indeed, patients with PAH had a mean LAS score of 53, which was even higher than for the IPF patients (74).
Several risk factors for worse outcome have been identified in PAH, such as the etiology of the PAH, male sex, older age, worse functional class, 6-minute walking distance, hemodynamic parameters, BNP and NT-proBNP values, etc. (75,76). These and other risk factors are used in the REVEAL scoring system, which is a quantitative equation for predicting survival and was prospectively validated in a cohort of newly diagnosed PAH patients from the REVEAL registry (77). Referral and transplantation guidelines are summarized in Table 5.
Conclusions
Guidelines for referral and transplantation are only guidelines and not an exact science. These guidelines give an idea when to think about lung transplantation and serve to refer a patient in time for transplantation. Of course, difficult situations remain and will not be solved by these guidelines. In general, we always ask our referring physicians to discuss every potential lung transplant candidate with the transplant center before referral. Also, a lot of decisions will depend upon the local waiting list, waiting times and outcomes of lung transplantation in a specific center. This is especially the case in so called difficult indications for lung transplantation (colonization with highly resistant bacteria, as discussed under CF, patients with scleroderma and severe reflux/dysmotility of the esophagus, etc.), which always need to be discussed with the transplant center before taking any decision.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yusen RD, Edwards LB, Dipchand AI, et al. Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016;35:1170-84. [Crossref] [PubMed]
- Maurer JR, Frost AE, Estenne M, et al. International guidelines for the selection of lung transplant candidates. The International Society for Heart and Lung Transplantation, the American Thoracic Society, the American Society of Transplant Physicians, the European Respiratory Society. J Heart Lung Transplant 1998;17:703-9. [PubMed]
- Maurer JR, Frost AE, Estenne M, et al. International guidelines for the selection of lung transplant candidates. The International Society for Heart and Lung Transplantation, the American Thoracic Society, the American Society of Transplant Physicians, the European Respiratory Society. Heart Lung 1998;27:223-9. [PubMed]
- Maurer JR, Frost AE, Estenne M, et al. International guidelines for the selection of lung transplant candidates. The International Society for Heart and Lung Transplantation, the American Thoracic Society, the American Society of Transplant Physicians, the European Respiratory Society. Transplantation 1998;66:951-6. [Crossref] [PubMed]
- Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745-55. [Crossref] [PubMed]
- Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34:1-15. [Crossref] [PubMed]
- Barshes NR, DiBardino DJ, McKenzie ED, et al. Combined lung and liver transplantation: The United States experience. Transplantation 2005;80:1161-7. [Crossref] [PubMed]
- Ceulemans LJ, Strypstein S, Neyrinck A, et al. Combined liver-thoracic transplantation: single-center experience with introduction of the 'Liver-first' principle. Transpl Int 2016;29:715-26. [Crossref] [PubMed]
- Ceulemans LJ, Monbaliu D, Verslype C, et al. Combined liver and lung transplantation with extended normothermic lung preservation in a patient with end-stage emphysema complicated by drug-induced acute liver failure. Am J Transplant 2014;14:2412-6. [Crossref] [PubMed]
- Bertani A, Grossi P, Vitulo P, et al. Successful lung transplantation in an HIV- and HBV-positive patient with cystic fibrosis. Am J Transplant 2009;9:2190-6. [Crossref] [PubMed]
- Fong TL, Cho YW, Hou L, et al. Outcomes after lung transplantation and practices of lung transplant programs in the United States regarding hepatitis C seropositive recipients. Transplantation 2011;91:1293-6. [Crossref] [PubMed]
- Dobbels F, De Bleser L, Berben L, et al. Efficacy of a medication adherence enhancing intervention in transplantation: The MAESTRO-Tx trial. J Heart Lung Transplant 2017;36:499-508. [Crossref] [PubMed]
- Ruttens D, Verleden SE, Goeminne PC, et al. Smoking resumption after lung transplantation: standardised screening and importance for long-term outcome. Eur Respir J 2014;43:300-3. [Crossref] [PubMed]
- Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:965-78. [Crossref] [PubMed]
- Plöchl W, Pezawas L, Artemiou O, et al. Nutritional status, ICU duration and ICU mortality in lung transplant recipients. Intensive Care Med 1996;22:1179-85. [Crossref] [PubMed]
- Lederer DJ, Wilt JS, D'Ovidio F, et al. Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med 2009;180:887-95. [Crossref] [PubMed]
- Ruttens D, Verleden SE, Vandermeulen E, et al. Body mass index in lung transplant candidates: a contra-indication to transplant or not? Transplant Proc 2014;46:1506-10. [Crossref] [PubMed]
- Hirt SW, Rahimi A, Möller F, et al. Does previous thoracic surgery increase perioperative risk in lung transplantation? Transplant Proc 2001;33:3572-3. [Crossref] [PubMed]
- Hayanga JW, Lira A, Aboagye JK, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation: what lessons might we learn from volume and expertise? Interact Cardiovasc Thorac Surg 2016;22:406-10. [Crossref] [PubMed]
- Biscotti M, Gannon WD, Agerstrand C, et al. Awake Extracorporeal Membrane Oxygenation as Bridge to Lung Transplantation: A 9-Year Experience. Ann Thorac Surg 2017;104:412-9. [Crossref] [PubMed]
- Lobo LJ, Chang LC, Esther CR Jr, et al. Lung transplant outcomes in cystic fibrosis patients with pre-operative Mycobacterium abscessus respiratory infections. Clin Transplant 2013;27:523-9. [Crossref] [PubMed]
- Smibert O, Snell GI, Bills H, et al. Mycobacterium abscessus Complex - a Particular Challenge in the Setting of Lung Transplantation. Expert Rev Anti Infect Ther 2016;14:325-33. [Crossref] [PubMed]
- Olland A, Falcoz PE, Kessler R, et al. Should cystic fibrosis patients infected with Burkholderia cepacia complex be listed for lung transplantation? Interact Cardiovasc Thorac Surg 2011;13:631-4. [Crossref] [PubMed]
- Quon BS, Reid JD, Wong P, et al. Burkholderia gladioli - a predictor of poor outcome in cystic fibrosis patients who receive lung transplants? A case of locally invasive rhinosinusitis and persistent bacteremia in a 36-year-old lung transplant recipient with cystic fibrosis. Can Respir J 2011;18:e64-5. [Crossref] [PubMed]
- Kern RM, Seethamraju H, Blanc PD, et al. The feasibility of lung transplantation in HIV-seropositive patients. Ann Am Thorac Soc 2014;11:882-9. [Crossref] [PubMed]
- Sweet MP, Herbella FA, Leard L, et al. The prevalence of distal and proximal gastroesophageal reflux in patients awaiting lung transplantation. Ann Surg 2006;244:491-7. [PubMed]
- Palmer SM, Miralles AP, Howell DN, et al. Gastroesophageal reflux as a reversible cause of allograft dysfunction after lung transplantation. Chest 2000;118:1214-7. [Crossref] [PubMed]
- Young LR, Hadjiliadis D, Davis RD, et al. Lung transplantation exacerbates gastroesophageal reflux disease. Chest 2003;124:1689-93. [Crossref] [PubMed]
- Shah N, Force SD, Mitchell PO, et al. Gastroesophageal reflux disease is associated with an increased rate of acute rejection in lung transplant allografts. Transplant Proc 2010;42:2702-6. [Crossref] [PubMed]
- Blondeau K, Mertens V, Vanaudenaerde BA, et al. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J 2008;31:707-13. [Crossref] [PubMed]
- Meyer KC, Raghu G, Verleden GM, et al. ISHLT/ATS/ERS BOS Task Force Committee. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 2014;44:1479-503. [Crossref] [PubMed]
- Hartwig MG, Anderson DJ, Onaitis MW, et al. Fundoplication after lung transplantation prevents the allograft dysfunction associated with reflux. Ann Thorac Surg 2011;92:462-8. [Crossref] [PubMed]
- Gulack BC, Meza JM, Lin SS, et al. Reflux and allograft dysfunction: is there a connection? Thorac Surg Clin 2015;25:97-105. [Crossref] [PubMed]
- Verleden GM, Vos R, Vanaudenaerde B, et al. Current views on chronic rejection after lung transplantation. Transpl Int 2015;28:1131-9. [Crossref] [PubMed]
- Miele CH, Schwab K, Saggar R, et al. Lung Transplant Outcomes in Systemic Sclerosis with Significant Esophageal Dysfunction. A Comprehensive Single-Center Experience. Ann Am Thorac Soc 2016;13:793-802. [Crossref] [PubMed]
- Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant 2016;35:433-9. [Crossref] [PubMed]
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005-12. [Crossref] [PubMed]
- Marchand E. The BODE index as a tool to predict survival in COPD lung transplant candidates. Eur Respir J 2010;36:1494-5. [Crossref] [PubMed]
- Eskander A, Waddell TK, Faughnan ME, et al. BODE index and quality of life in advanced chronic obstructive pulmonary disease before and after lung transplantation. J Heart Lung Transplant 2011;30:1334-41. [Crossref] [PubMed]
- Lane CR, Tonelli AR. Lung transplantation in chronic obstructive pulmonary disease: patient selection and special considerations. Int J Chron Obstruct Pulmon Dis 2015;10:2137-46. [PubMed]
- Lahzami S, Bridevaux PO, Soccal PM, et al. Survival impact of lung transplantation for COPD. Eur Respir J 2010;36:74-80. [Crossref] [PubMed]
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925-31. [Crossref] [PubMed]
- Soler-Cataluña JJ, Martínez-García MA, Sánchez LS, et al. Severe exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med 2009;103:692-9. [Crossref] [PubMed]
- Connors AF Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Prefer ences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996;154:959-67. [Crossref] [PubMed]
- Tokgoz Akyil F, Gunen H, Agca M, et al. Patient Outcome after Chronic Obstructive Pulmonary Disease Exacerbations Requiring Non-invasive Ventilation during Hospitalization. Arch Bronconeumol 2016;52:470-6. [Crossref] [PubMed]
- National Emphysema Treatment Trial Research Group, Fishman A, Fessler H, et al. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075-83. [Crossref] [PubMed]
- Klooster K, Ten Hacken NH, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015;373:2325-35. [Crossref] [PubMed]
- Klooster K, Hartman JE, Ten Hacken NH, et al. One-Year Follow-Up after Endobronchial Valve Treatment in Patients with Emphysema without Collateral Ventilation Treated in the STELVIO Trial. Respiration 2017;93:112-21. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Wilson KC, Raghu G. The 2015 guidelines for idiopathic pulmonary fibrosis: an important chapter in the evolution of the management of patients with IPF. Eur Respir J 2015;46:883-6. [Crossref] [PubMed]
- Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;157:199-203. [Crossref] [PubMed]
- Thomeer MJ, Vansteenkiste J, Verbeken EK, et al. Interstitial lung diseases: Characteristics at diagnosis and mortality risk assessment. Respir Med 2004;98:567-73. [Crossref] [PubMed]
- Latsi PI, du Bois RM, Nicholson AG, et al. Fibrotic idiopathic interstitial pneumonia: The prognostic value of longitudinal functional trends. Am J Respir Crit Care Med 2003;168:531-7. [Crossref] [PubMed]
- Nathan SD, Shlobin OA, Weir N, et al. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest 2011;140:221-9. [Crossref] [PubMed]
- Zappala CJ, Latsi PI, Nicholson AG, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:830-6. [Crossref] [PubMed]
- Mogulkoc N, Brutsche MH, Bishop PW, et al. Pulmonary function in idiopathic pulmonary fibrosis and referral for lung transplantation. Am J Respir Crit Care Med 2001;164:103-8. [Crossref] [PubMed]
- Hanson D, Winterbauer RH, Kirtland SH, et al. Changes in pulmonary function test results after 1 year of therapy as predictors of survival in patients with idiopathic pulmonary fibrosis. Chest 1995;108:305-10. [Crossref] [PubMed]
- Simon-Blancal V, Freynet O, Nunes H, et al. Acute exacerbation of idiopathic pulmonary fibrosis: outcome and prognostic factors. Respiration 2012;83:28-35. [Crossref] [PubMed]
- Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265-75. [Crossref] [PubMed]
- Nadrous HF, Pellikka PA, Krowka MJ, et al. The impact of pulmonary hypertension on survival in patients with idiopathic pulmonary fibrosis. Chest 2005;128:616S-617S. [Crossref] [PubMed]
- Thabut G, Mal H, Castier Y, et al. Survival benefit of lung transplantation for patients with idiopathic pulmonary fibrosis. J Thorac Cardiovasc Surg 2003;126:469-75. [Crossref] [PubMed]
- Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011;377:1760-9. [Crossref] [PubMed]
- Noble PW, Albera C, Bradford WZ, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J 2016;47:243-53. [Crossref] [PubMed]
- Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med 2011;365:1079-87. [Crossref] [PubMed]
- Richeldi L, du Bois RM, Raghu G, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. [Crossref] [PubMed]
- ten Klooster L, Nossent GD, Kwakkel-van Erp JM, et al. Ten-Year Survival in Patients with Idiopathic Pulmonary Fibrosis After Lung Transplantation. Lung 2015;193:919-26. [Crossref] [PubMed]
- Delanote I, Wuyts WA, Yserbyt J, et al. Safety and efficacy of bridging to lung transplantation with antifibrotic drugs in idiopathic pulmonary fibrosis: a case series. BMC Pulm Med 2016;16:156. [Crossref] [PubMed]
- Vos R, Verleden GM, Dupont LJ. Long-term survival after lung transplantation among cystic fibrosis patients: Moving away from mere palliation. J Heart Lung Transplant 2016;35:837-40. [Crossref] [PubMed]
- Lonni S, Chalmers JD, Goeminne PC, et al. Etiology of Non-Cystic Fibrosis Bronchiectasis in Adults and Its Correlation to Disease Severity. Ann Am Thorac Soc 2015;12:1764-70. [Crossref] [PubMed]
- Kerem E, Reisman J, Corey M, et al. Prediction of mortality in patients with cystic fibrosis. N Engl J Med 1992;326:1187-91. [Crossref] [PubMed]
- De Soyza A, Meachery G, Hester KL, et al. Lung transplantation for patients with cystic fibrosis and Burkholderia cepacia complex infection: a single-center experience. J Heart Lung Transplant 2010;29:1395-404. [Crossref] [PubMed]
- Lobo LJ, Tulu Z, Aris RM, et al. Pan-Resistant Achromobacter xylosoxidans and Stenotrophomonas maltophilia Infection in Cystic Fibrosis Does Not Reduce Survival After Lung Transplantation. Transplantation 2015;99:2196-202. [Crossref] [PubMed]
- Parize P, Boussaud V, Poinsignon V, et al. Clinical outcome of cystic fibrosis patients colonized by Scedosporium species following lung transplantation: A single-center 15-year experience. Transpl Infect Dis 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Gottlieb J, Greer M, Sommerwerck U, et al. Introduction of the lung allocation score in Germany. Am J Transplant 2014;14:1318-27. [Crossref] [PubMed]
- Humbert M, Sitbon O, Yaici A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 2010;36:549-55. [Crossref] [PubMed]
- Raina A, Humbert M. Risk assessment in pulmonary arterial hypertension. Eur Respir Rev 2016;25:390-8. [Crossref] [PubMed]
- Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010;122:164-72. [Crossref] [PubMed]