Moving more potent and less toxic options to the frontline in the management of advanced lung cancer
Introduction
The most common primary lung cancers can be divided into small cell lung cancer and non-small cell lung cancer (NSCLC)—the latter compromised predominantly of adenocarcinoma and squamous cell carcinoma histologies.
In the last fifteen years, somatic genomic analysis of NSCLC has identified critical driver oncogenes in a subset of these tumors (1). In turn, the identification and delineation of these oncogenic events has led to the successful development of nearly ten different oral targeted therapies now approved for use in routine clinical practice for advanced NSCLCs (2-6). These are directed against: mutations in the epidermal growth factor receptor (EGFR) (7-10), anaplastic lymphoma kinase (ALK) (11-14) gene rearrangements, ROS proto-oncogene 1 (ROS1) (15) gene rearrangements, and B-Raf proto-oncogene, serine/threonine kinase (BRAF) mutations (16) (Figure 1). These well vetted targeted therapies have demonstrated superior and more durable clinical outcomes and quality of life in patients with genotype-defined advanced NSCLC, as compared to the efficacy and toxicity profiles associated with cytotoxic chemotherapies in this disease. Ongoing research also shows promising potential for use of kinase inhibitors for other putative driver mutations in NSCLC (17).

Immunotherapy can also be efficacious in solid tumors with high somatic mutational burden, including NSCLC (18-20). The most promising examples include multiple anti-programmed cell death protein 1 (PD-1)/anti-programmed death-ligand 1 (PD-L1) immune checkpoint inhibitors. Both types of immune checkpoint inhibitors have been demonstrated in rigorous, phase III studies to improve objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) in certain patients with advanced NSCLC when compared to standard cytotoxic chemotherapy (18,21,22). Anti-PD-1/PD-L1 antibodies are now used as both first and second line therapies for advanced NSCLC, depending on the tumor’s PD-L1 tumor proportion score (TPS) as assessed by immunohistochemistry (IHC) (Figure 1).
Thus, the initial evaluation and management of advanced NSCLC and use of biomarkers for therapeutic stratification has evolved substantially over the past decade plus and with progressive and important improvements in clinical outcomes and quality of life for patients living with this disease. In our own cancer center, we adhere to the diagnostic and treatment pathway described in Figure 1 for advanced NSCLCs. This scheme incorporates evolving worldwide drug approval patterns and evidence-/guideline-based treatment strategies (23).
ALK and the development of the first approved ALK inhibitor crizotinib
The ALK gene is located on chromosome 2p in humans (24). The first ALK rearrangements identified in NSCLC were small inversions within chromosome 2p leading to the formation of a fusion gene comprising portions of the echinoderm microtubule-associated protein-like 4 (EML4) and ALK. These fusion translocations were noted to be oncogenic in preclinical models (5,25,26). By 2007, ALK rearrangements were established as bona fide oncogenic drivers in NSCLC and potential targets for development of tyrosine kinase inhibitors (TKIs).
Crizotinib (formerly PF-02341066, Pfizer, New York, USA) is a small molecule TKI that was originally developed to target MET proto-oncogene, receptor tyrosine kinase (MET) prior to the discovery of ALK rearrangements in NSCLC (27). It is now known to be multi-targeted TKI, with efficacy against MET, ALK, and ROS1 kinase domains (28). The first-in-human clinical trial of crizotinib commenced before the seminal report of ALK-rearranged NSCLC and allowed the first glimpse of activity in patients whose tumors harbor ALK rearrangements. This clinical trial (NCT00585195, also known as PROFILE_1001) established crizotinib at a dose of 250 mg orally twice daily for the treatment of ALK-rearranged NSCLC. Crizotinib has adequate tolerability in most patients, with nausea, diarrhea, and mild visual disturbance as the most common adverse events. Impressively, in the initial 82 patients with ALK rearrangements treated in this study, the reported ORR was 57% (11,29). Based on rapid and sustained responses, crizotinib received accelerated approval for advanced ALK-rearranged NSCLC in August 2011. By 2013, a randomized trial (PROFILE_1007, NCT00932893) in the second line setting established improved ORR and PFS in this subset of patients receiving crizotinib as compared to cytotoxic chemotherapy (30). PROFILE_1014 (NCT01154140) subsequently established crizotinib as the standard of care for first line palliative therapy in ALK-rearranged advanced NSCLC. In this trial, 343 patients were randomized to receive crizotinib or conventional platinum doublet chemotherapy in the first line setting (31). ORR and PFS were superior for crizotinib vs. platinum doublet, with a median PFS of 10.9 vs. 7.0 months, respectively. These results have firmly established the use of crizotinib as first line therapy for TKI-naïve advanced ALK-rearranged NSCLC since 2014.
Acquired resistance to crizotinib and development of next generation ALK inhibitors
Despite the significant clinical success with crizotinib in the advanced disease setting, acquired resistance occurs almost inevitably within the initial year of use. Our cancer center’s own longitudinal cohort established that <10% of cases reach three or more years of crizotinib monotherapy without the identification of tumor progression (32). The two most common mechanisms that lead to disease progression in crizotinib-treated tumors include: (I) re-activation of ALK signaling by acquired secondary mutations in the ALK kinase domain or (II) pharmacokinetic escape - usually manifested as central nervous system (CNS) disease progression (33-37). To address these clinical challenges, a variety of potent ALK TKIs are under development. To date, three next generation ALK inhibitors—ceritinib, brigatinib, and alectinib—have gained regulatory approval (Figure 1).
Ceritinib (formerly LDK378, Novartis, Basel, Switzerland) was developed as a TKI with preclinical activity against ALK and ROS1 fusion proteins (12,38-40). Ceritinib is about 10 times as potent as crizotinib and is able to inhibit many crizotinib-resistant ALK mutants, including the gatekeeper ALK-L1196M mutant (38). The phase I clinical trial of ceritinib (ASCEND, NCT01283516) highlighted that gastrointestinal adverse events (nausea, diarrhea, and vomiting) established the maximum tolerated dose (MTD) (12). Nevertheless, the efficacy of ceritinib was established with an ORR of 56% in previously treated patients and 62% in TKI-naïve ALK-rearranged advanced NSCLCs (12). Most importantly, responses were seen in tumors that harbored ALK mutations (such as L1196M, 1151Tins, S1206Y and G1269A), lacked known mechanisms of resistance to crizotinib, and in those patients with brain metastases (12,41,42). Ceritinib received regulatory approval for use in crizotinib-resistant or intolerant patients in 2014.
Brigatinib (formerly AP26113, Ariad/Takeda, Deerfield, USA) is another potent ALK TKI with a broader spectrum of activity against ALK kinase domain mutants. Clinical data from the brigatinib phase II ALTA clinical trial (NCT02094573) showed ORRs of 45% and 54% for doses of 90 and 180 mg daily, respectively (14). The most noticeable adverse event associated with brigatinib is early pulmonary toxicity. Subsequent study schema have therefore incorporated a dose escalation phase with a starting dose of 90 mg orally daily for 7 days, then increasing to 180 mg daily. Outside of pulmonary concerns, the drug is otherwise well tolerated and with serious gastrointestinal side effects and headaches occurring in a minority of cases (14). Brigatinib received regulatory approval for use in crizotinib-resistant or intolerant patients in 2017.
Alectinib (formerly CH5424802, Roche/Chugai, Basel, Switzerland/Tokyo, Japan) is a selective ALK inhibitor. Similar to other next generation ALK TKIs, it has greater potency and higher CNS penetration than crizotinib and is active against ALK fusion proteins with ALK kinase domain mutants (13,43). In the initial Japanese phase I/II study (AF-001JP), alectinib was highly active (44). The drug was extremely well tolerated with minimal serious adverse events. Constipation, fatigue and peripheral edema were the most common adverse events. These findings led to the approval of alectinib in Japan in July 2014. Worldwide, alectinib is prescribed at 600 mg twice daily and was approved in 2015 following results from two single-arm phase-II trials [global NP28673 (NCT01801111) and the North American NP28761 (NCT01871805) studies] in patients with disease progression on or intolerance to crizotinib. These studies confirmed ORRs of approximately 50% or higher for systemic and intracranial sites of tumor burden (13,45).
J-ALEX and ALEX clinical trials confirm superiority of alectinib when compared to crizotinib
Given the promising efficacy and tolerability of alectinib in the AF-001JP study, a phase III trial (J-ALEX, JapicCTI-132316) was designed to directly compare the efficacy and safety of alectinib 300 mg twice daily versus crizotinib 250 mg twice daily in Japanese patients with advanced ALK-rearranged NSCLC (46). Between November 2013 and August 2015, 207 patients were enrolled and randomly assigned to receive alectinib (n=103) or crizotinib (n=104). At the second planned interim analysis, alectinib met the trials primary endpoint of superiority in PFS [hazard ratio (HR) of 0.34 (99.7% CI: 0.17–0.71), P<0.0001]. After about 12 months of follow-up in each of the treatment groups, median PFS had not yet been reached with alectinib (95% CI: 20.3 to not reached) and was 10.2 months (95% CI: 8.2–12.0 months) with crizotinib. Furthermore, alectinib showed better tolerability with less serious adverse events and less frequent dose interruptions as compared to crizotinib.
J-ALEX’s data was further supported by the multi-center international ALEX trial (NCT02075840), comparing alectinib 600 mg twice daily versus crizotinib 250 mg twice daily in TKI-naïve, ALK-rearranged advanced NSCLC (47). Again, the median PFS (primary outcome) with alectinib was not reached (95% CI: 17.7 months to not reached) as compared with 11.1 months (95% CI: 9.1–13.1 months) with crizotinib (HR of 0.47 95% CI: 0.34–0.65; P<0.001). These two studies were the first trials to compare head-to-head the efficacy and safety of a next generation ALK inhibitor to crizotinib (46,47). The trials convincingly show that alectinib is superior in prolonging median PFS with lesser toxicity than crizotinib. Although with relatively short follow up, the OS of first line alectinib to date remains non-inferior to crizotinib (46,47).
Given the significant impact of intracranial disease burden on the long-term outcomes of patients with ALK-rearranged advanced NSCLC, it is important to note that both trials evaluated alectinib’s efficacy in patients with CNS metastases. The J-ALEX trial had a pre-planned exploratory analysis of cumulative incidence of CNS disease with competing events of death, CNS progression, and non-CNS progression. The results indicate that alectinib reduced the risk of progression in both non-CNS and CNS lesions compared with crizotinib (46). For the ALEX trial, an independent analysis conducted solely for assessment of CNS disease showed that the time to CNS progression was significantly longer with alectinib than with crizotinib (cause-specific HR of 0.16, 95% CI: 0.10–0.28). The rate of events (CNS progression) was only 12% with alectinib vs. 45% with crizotinib during the duration of follow-up (47). The intracranial ORR in the alectinib group was also higher than in the crizotinib group, thus establishing alectinib as an ALK TKI with superior ability to control existing and delay subsequent intracranial disease, something that crizotinib had been previously shown to do when compared to platinum-based chemotherapy (34,47,48).
Prolonged durations of PFS in the TKI-naïve setting have also been reported with the use of ceritinib within the ASCEND-4 trial (NCT01828099, ceritinib versus platinum doublet) (42). Median PFS was 16.6 months (95% CI: 12.6–27.2 months) with ceritinib at a starting dose of 750 mg daily. Common dose-limiting toxicities include: diarrhea (85% of cases), nausea (69% of cases), and vomiting (66% cases). Ceritinib gained additional approval for first line use in ALK rearranged advanced NSCLC in 2017.
Ongoing trials, future directions and conclusions
Outcomes from the pivotal J-ALEX and ALEX trials have revamped the paradigm for first line management of advanced ALK-rearranged NSCLCs. In moving more potent next generation ALK TKIs to the frontline setting, superior clinical efficacy—particularly with regards to intracranial disease—along with improved tolerability have been hallmarks of the continued advancement and drug development in this subset of advanced lung cancers (Figure 2). In parallel to these developments in the ALK-directed arena, outcomes from the ongoing FL-AURA trial (NCT02296125) comparing the 3rd generation EGFR TKI osimertinib to the 1st generation EGFR TKIs erlotinib and gefitinib in the first line setting may well change the benchmark for initial management of EGFR-mutated NSCLC, as well. This trial has completed accrual, and results are awaited later this year.
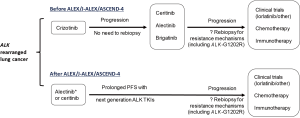
The main limitation of next generation ALK TKIs (alectinib, ceritinib, brigatinib, and other ALK TKIs) is the inevitable development of acquired resistance—particularly through selection of clones with ALK kinase domain mutations (49). More than half of alectinib- and ceritinib-resistant tumors develop the ALK-G1202R mutation which confers a high degree of cross-resistance to all other approved ALK TKIs (37,49). However, recent investigation of the more potent TKI lorlatinib and other in-development ALK inhibitors suggest that this resistance profile may be overcome (49,50). The completed phase I/II clinical trial of lorlatinib (NCT01970865) is undergoing evaluation by regulatory agencies, and the drug may be soon approved for patients following failure of one or more prior ALK TKIs (51). Another ongoing trial is evaluating lorlatinib vs. crizotinib in the TKI-naive first line setting (NCT03052608). It is possible that with early use of more potent ALK inhibitors (i.e., lorlatinib), patterns of ALK TKI resistance may become dominated by bypass pathway upregulation rather than ALK kinase domain resistance mutations (49). Therefore, combination strategies may be required to prevent or overcome this type of resistance (33,37,52).
ALK-rearranged lung cancer has been exemplary in the field of targeted cancer therapy in many ways. In the span of 4 years between 2007 and 2011, EML4-ALK went from a just discovered driver oncogene in NSCLC to a readily actionable target with approval of crizotinib, a highly effective and tolerable therapy. Next, evolution in the understanding of ALK-dependent and pharmacokinetic mechanisms of resistance to crizotinib have paved the way for the clinical development of next generation ALK TKIs ceritinib [2014], alectinib [2015], and brigatinib [2017]. Most recently, the J-ALEX and ALEX trials have established that the more potent, less toxic next generation ALK TKI alectinib should now supersede crizotinib as the evidence-based standard for first line palliative therapy in TKI-naïve, advanced ALK-rearranged NSCLC (Figure 2) in countries that grant regulatory approval for this indication.
Acknowledgements
None.
Footnote
Conflicts of Interest: DB Costa has received consulting fees and honoraria from Pfizer, Boehringer Ingelheim and Ariad pharmaceuticals; outside the submitted work. Other authors have no conflicts of interest to declare.
References
- Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012;150:1107-20. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Yasuda H, Figueiredo-Pontes LL, Kobayashi S, et al. Preclinical Rationale for Use of the Clinically Available Multitargeted Tyrosine Kinase Inhibitor Crizotinib in ROS1-Translocated Lung Cancer. J Thorac Oncol 2012;7:1086-90. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma With EGFR Mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234-42. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490-8. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-Rearranged Non-Small-Cell Lung Cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Planchard D, Besse B, Groen HJ, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984-93. [Crossref] [PubMed]
- Soo RA, Stone EC, Cummings KM, et al. Scientific Advances in Thoracic Oncology 2016. J Thorac Oncol 2017;12:1183-209. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Borghaei H, Brahmer J. Nivolumab in Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2016;374:493-4. [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Shaw AT, Engelman JA. ALK in Lung Cancer: Past, Present, and Future. J Clin Oncol 2013;31:1105-11. [Crossref] [PubMed]
- Soda M, Takada S, Takeuchi K, et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci U S A 2008;105:19893-7. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Christensen JG, Zou HY, Arango ME, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther 2007;6:3314-22. [Crossref] [PubMed]
- Jorge SE, Schulman S, Freed JA, et al. Responses to the multitargeted MET/ALK/ROS1 inhibitor crizotinib and co-occurring mutations in lung adenocarcinomas with MET amplification or MET exon 14 skipping mutation. Lung Cancer 2015;90:369-74. [Crossref] [PubMed]
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Rangachari D, Le X, Shea M, et al. Cases of ALK-Rearranged Lung Cancer with 5-Year Progression-Free Survival with Crizotinib as Initial Precision Therapy. J Thorac Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Costa DB. Resistance to ALK inhibitors: Pharmacokinetics, mutations or bypass signaling? Cell Cycle 2017;16:19-20. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [Crossref] [PubMed]
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4:120ra17. [Crossref] [PubMed]
- Costa DB. Ascending role of next-generation ALK inhibitors. Lancet Oncol 2017;18:837-9. [Crossref] [PubMed]
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014;4:662-73. [Crossref] [PubMed]
- Gainor JF, Tan DS, De PT, et al. Progression-Free and Overall Survival in ALK-Positive NSCLC Patients Treated with Sequential Crizotinib and Ceritinib. Clin Cancer Res 2015;21:2745-52. [Crossref] [PubMed]
- Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Shaw AT, Kim TM, Crino L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18:874-86. [Crossref] [PubMed]
- Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol 2013;14:590-8. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial Efficacy of Crizotinib Versus Chemotherapy in Patients With Advanced ALK-Positive Non-Small-Cell Lung Cancer: Results From PROFILE 1014. J Clin Oncol 2016;34:2858-65. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med 2016;374:54-61. [Crossref] [PubMed]
- Shaw AT, Ou SH, Felip E, et al. Efficacy and safety of lorlatinib in patients (pts) with ALK+ non-small cell lung cancer (NSCLC) with one or more prior ALK tyrosine kinase inhibitor (TKI): A phase I/II study. J Clin Oncol 2017;35:(suppl; abstr 9006).
- Costa DB. ALK inhibitors: plateauing systemic and intracranial activity? Lancet Oncol 2016;17:404-6. [Crossref] [PubMed]


