Rethinking the role of postoperative critical care in an inequitable world
Inpatient care is a concept that is likely to be as old as medicine itself. Infirmaries and hospitals were established as places for providing better care for the sick, and often to isolate and keep them away in order to protect the community. In such establishments, it would have been logical to arrange patients in a way that those who were sicker would be more at sight to receive better care from the staff. During the Crimean War in the mid-19th century, Florence Nightingale placed the more severely injured soldiers near the equivalent of today’s “nursing station” to be able to provide them with “more intensive nursing care”. In the early 20th century, Johns Hopkins Hospital hosted one of the first postoperative intensive care units that was staffed by specialized nurses under supervision of surgeons to care for patients having neurosurgery. This concept was further developed and popularized leading to the establishment of postoperative recovery units during the Second World War (1). Eventually in the 1950s and following the Copenhagen polio epidemic that left over 300 patients in need of around-the-clock respiratory support—provided heroically by hundreds of medical and dental students using rubber bags connected to tracheostomy tubes—the first Intensive Care Units (ICUs) were established in Europe and across the globe, formally launching the era of intensive care medicine (2).
Fast forward 60 years and today, ICUs are a fixture of hospitals everywhere—well almost everywhere. According to the World Health Organization (WHO), out of over $7.5 trillion global health care expenditure in 2014, $46 was the average share of someone living in one of the least developed countries (as classified by the United Nations), compared with $8,990 for someone fortunate to be living in North America. While more spending on health does not necessarily mean a better outcome, this wide spread in health expenditure translates into a staggering disparity in access to health care across the globe (3).
As a pinnacle of inpatient care, ICUs are among the most costly and demanding hospital units to launch and operate, making their availability even more challenging in the developing nations and underserved areas, and further contributing to the despicable “10/90 gap” affecting the health care globally (4). Reports of availability range from less than 1 to more than 30 ICU beds per 100,000 people, although variations on how ICU beds are defined, staffing requirements and target admitting criteria can further exaggerate these figures. We should bear in mind that these numbers are national averages and local access to critical care services within each country can vary even more (5). A country might have ICU bed shortage, but even those few beds can be disproportionally concentrated in major cities, leaving all others unable to care for the critically ill.
With availability so disparate, one key question to ask is the impact of ICU access on quality of care and clinical outcomes of various patient populations. Data from the United Kingdom—with a disproportionally low number of ICU beds compared with other countries of similar socioeconomic status—suggests that the shortage can lead to denied admission of needy patients and premature discharge of patients from ICU, while addition of more ICU beds across the country was associated with reduced mortality (5). On the other hand, too much of a good thing is not necessarily better. When Robert et al. compared ICUs with usually high bed availability versus those with low bed availability, they observed that availability of more beds was associated with significantly more frequent admission of patients who were less likely to benefit from ICU care (those too well, or too sick to benefit) (6). Perhaps this is a reflection of human nature that when a resource is limited, we would think twice before utilizing it.
Patients undergoing surgery under general anesthesia are often considered to be at increased risk of suffering from complications during the perioperative period. This has led some to propose routine admission of these patients in ICU following the surgery. The idea is that being under the closer watch of an ICU would permit an earlier diagnosis and better management of any potential complications. Given the limited access to ICUs, does this costly routine help patients to achieve better outcomes?
Recently, Kahan et al. studied the association between ICU admission following surgery and in-hospital mortality among over 44,000 patients from 474 hospitals across 27 countries in 2014 as part of the International Surgical Outcome Study (ISOS) (7). The ISOS investigators and the authors should be commended on undertaking such a substantial endeavor, as they collected data prospectively form 99% of the eligible patients—adults scheduled for elective surgery with planned overnight hospital stay—admitted in each of the participating centers within a pre-designated 7-day period. The central idea behind the study was that comparison of mortality rates among cohorts of patients who were admitted to ICUs right after surgery and those who were not could provide evidence on the impact of ICU provision on the outcomes of patients. The data were analyzed at patient- and hospital-level with adjustment for some potential confounders, with further comparisons between the high income and low or middle income countries (7).
The key finding of this study was that while the crude and adjusted mortality rates of patients who were admitted to ICU were significantly higher than those admitted to the standard wards right after the surgery, there was no association between the risk-adjusted mortality rate and percentage of admission to ICU at the hospital-level (7). The first observation—increased mortality among ICU-destined patients—is not unexpected, since patients who end up in ICU are generally sicker to begin and this is supported by the comparison of the baseline data in this study (e.g., higher preoperative ASA scores and rate of co-morbidities in patients who were admitted to ICU). A proper comparator would be a group of patients with similar level of acuity and burden of disease who were not admitted to an ICU (for example, due to access issues).
In an ideal world (for statisticians and not necessarily for the patients), one could imagine a controlled trial to randomly assign patients who are being considered for ICU admission to care at ICU versus standard ward and compare the outcomes. Needless to say, such a design faces ethical issues related to withholding of potentially life-saving care from needy patients (a scenario that sounds very familiar to us in context of another hotly debated common intervention—blood transfusion). In both cases, the enthusiastic investigators are faced with two options: wait indefinitely for the ultimate randomized trial that might never be conducted, or look at what is feasible now and try to make the best out of it (8).
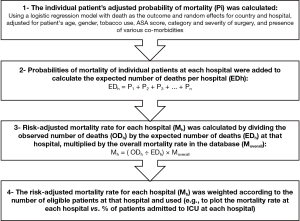
In our opinion, Kahan et al. have made the right choice in pursuing an answer for this important question based on observational data. While their study provides highly valuable insights on the clinical value of ICU admission following surgery, its results should be interpreted in light of the limitations of the study. To appreciate the limitations, it is important to understand the methodology for their hospital-level analysis. Figure 1 depicts a simplified view of their statistical methodology. We believe that the crux of their approach lies in their ability to model and calculate the adjusted mortality rate of each patient (Step 1 in Figure 1). If the model is robust and has a high predictive value (e.g., capable of predicting deaths using the available factors with great success), the rest of the steps can produce fairly reliable results, as the mortality rate at each hospital is adjusted for the variations in the baseline risk of the patients. If however the model does not have a high predictive value, the validity of the rest of the steps can be severely undermined since the baseline risk of the patients could not have been accounted for. The authors did not provide any measures of the overall performance of their regression models for mortality, and hence we cannot be quite sure how reliable the results are.
Their study focused on in-hospital 30-day mortalities, indeed an important outcome measure, but one that is at the end of a spectrum of various morbidities and it might not be as informative as other (more common) measures. Will our conclusion from this study stay the same if we learn that despite comparable 30-day mortality rates, one cohort would be far more likely to suffer a major cardiac or renal complication or have worse quality of life after discharge?
The authors defined ICU as a facility that routinely admits patients in need of invasive ventilation overnight, although the lack of a unified definition for what constitutes an ICU is well documented (5). Lack of standardized admission criteria for postoperative ICU is another issue to remember. Since admission to ICU was the defining event for the study cohorts, it is critical to determine if a patient was admitted to ICU as a routine practice, or perhaps because the clinicians had patient-specific reasons to call for ICU admission. The fact that the investigators also included am analysis of mortality and ICU admission to treat a postoperative complication indicates that not all ICU admissions were routine. These and other issues can undermine the results and conclusions of this study (7).
The provided data on the individual centers are limited in this study. An important example is the nurse-to-patient ratio in the wards or in the ICUs, which can vary from hospital to hospital and may impact patient care. Also, there is no indication whether some ICUs were specialized (e.g., cardiothoracic or neurological ICUs, given that patients undergoing cardiothoracic or head and neck procedures were included) and who oversaw patient care. For example, were Intensivists involved or was the care provided primarily by the relevant specialists related to the procedure, such as cardiac surgeon or neurosurgeon. Other important yet missing information included whether the ICUs were closed or open and whether they have designated ICU teams, such as full time Intensivists, house-staff and midlevel practitioners rendering care. Were the Intensivists present in the ICUs around the clock or for only a part of the day? These are important questions and issues, which might have direct impact on patient care and outcomes.
The study by Kahan et al. provides some pleasant surprises. The reported median critical care capacity (defined as the ratio of ICU beds to the total number of hospital beds) was 2.8% in low and middle income countries vs. 3.6% in high income countries, suggestive of the narrowing disparities between the haves and have-nots. Of course one can still argue that the ratios alone will not provide a holistic picture of the regional access to care, if not adjusted for the target population numbers: two hospitals with 3 ICU beds out of a total of 100 beds would have the same critical care capacity of 3%, but one could be serving a small town of 10,000 while the other can be the only inpatient facility covering a population of 100,000 or more, making the effective access to ICU 10-fold less.
Where are we heading from here? A recent study using a novel measure of health care access across 195 countries and territories called the Healthcare Quality and Access (HAQ) index indicates that while the HAQ index improved for almost all countries from 1990 to 2015, the gap between the highest and lowest HAQ indexes widened as well (9). In short, as a species we are getting better in providing access to health care at a global level, but we are not as successful in battling the inequity in health care access yet. Similar studies focusing on global burden of critical illness and access to critical care are needed (10,11).
In the meanwhile and while we should continue to battle the inequities in health care and critical care, the role of ICU as a finite resource in short supply should be remembered. If we take the findings of the study by Kahan et al. at face value (7), the observed lack of benefit for ICU admission following surgery can be attributed to the poor selection and triage of the patients. Like all other medical treatments and management strategies, evidence-based guidelines and indications should direct the decision making process to make sure only patients who are likely to benefit from the intervention are selected for ICU admission.
A task force has recently attempted to update the Society of Critical Care Medicine’s guidelines for ICU admission, discharge and triage (12). With regards to postoperative care, the task force suggested that “patients with risk factors for postoperative instability or decompensation (should) be closely monitored and managed in a higher level of care unit than the ward in the immediate postoperative period.” The task force concluded that while complex postoperative patients can benefit from ICU admission, routine surgical patients may still be adequately monitored and managed out of ICU by an adequately trained nursing staff. In their ICU Admission Prioritization Framework, they assigned priority rank of 3 (on a scale of 1 to 5) to these patients and recommended an intermediate medical unit (IMU) level of care. Caring for these patients out of ICU can certainly open up the precious space in ICU for much more needy patients, but it should be noted that the task force rated its recommendation on these patients as “ungraded” underscoring the paucity of evidence and need for more investigation (12). While not conclusive, the study by Kahan et al. provides an important piece of evidence in this regard and makes a significant contribution to efforts to improve the utilization of ICUs across the globe in order to achieve the best overall outcome for the greatest number of patients while ensuring the provision of standard care for every single patient.
Acknowledgements
None.
Footnote
Conflicts of Interest: A Shander has been a consultant or speaker with honorarium for or received research support from, Masimo, Gauss, and Vifor; He is a founding member of the Society for the Advancement of Blood Management (SABM). M Javidroozi has been a consultant and contractor for SABM and Gauss Surgical. C Gianatiempo declares no relevant conflict of interests.
References
- Ristagno G, Weil MH. History of Critical Care Medicine: The Past, the Present and the Future. In: Gullo A, Lumb PD, Besso J, et al. editors. Intensive and Critical Care Medicine: WFSICCM World Federation of Societies of Intensive and Critical Care Medicine. Milano: Springer Milan, 2009:3-17.
- Kelly FE, Fong K, Hirsch N, Nolan JP. Intensive care medicine is 60 years old: the history and future of the intensive care unit. Clin Med (Lond) 2014;14:376-9. [Crossref] [PubMed]
- Global Health Expenditure Database. World Health Organization. Available online: http://apps.who.int/nha/database. Last Updated: 8-4-2017. Accessed: 8-29-2017.
- Fowler RA, Adhikari NK, Bhawanjee S. Clinical review: critical care in the global context--disparities in burden of illness, access, and economics. Crit Care 2008;12:225. [Crossref] [PubMed]
- Prin M, Wunsch H. International comparisons of intensive care: informing outcomes and improving standards. Curr Opin Crit Care 2012;18:700-6. [Crossref] [PubMed]
- Robert R, Coudroy R, Ragot S, et al. Influence of ICU-bed availability on ICU admission decisions. Ann Intensive Care 2015;5:55. [Crossref] [PubMed]
- Kahan BC, Koulenti D, Arvaniti K, et al. Critical care admission following elective surgery was not associated with survival benefit: prospective analysis of data from 27 countries. Intensive Care Med 2017;43:971-9. [Crossref] [PubMed]
- Trentino K, Farmer S, Gross I, et al. Observational studies - should we simply ignore them in assessing transfusion outcomes? BMC Anesthesiol 2016;16:96. [Crossref] [PubMed]
- GBD 2015 Healthcare Access and Quality Collaborators. Healthcare Access and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990-2015: a novel analysis from the Global Burden of Disease Study 2015. Lancet 2017;390:231-66. [Crossref] [PubMed]
- Adhikari NK, Rubenfeld GD. Worldwide demand for critical care. Curr Opin Crit Care 2011;17:620-5. [Crossref] [PubMed]
- Vincent JL, Marshall JC, Namendys-Silva SA, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med 2014;2:380-6. [Crossref] [PubMed]
- Nates JL, Nunnally M, Kleinpell R, et al. ICU Admission, Discharge, and Triage Guidelines: A Framework to Enhance Clinical Operations, Development of Institutional Policies, and Further Research. Crit Care Med 2016;44:1553-602. [Crossref] [PubMed]


