Pulmonary metastasectomy in uterine malignancies: outcome and prognostic factors
Introduction
Uterine malignancies (UM) include different histologies with different behaviors and outcomes. Endometrial adenocarcinoma (EA) is the most frequent type followed by papillary serous adenocarcinoma (PSA) and uterine leiomyosarcoma (UL). According to Surveillance, Epidemiology, and End Results (SEER) (1) the percentage of new cases of endometrial cancer (EC) compared to all cancers every year is 3.6% with a rate of death equal to 1.8%. Squamous cell carcinoma (SCC) represents the most frequent cancer of the uterine cervix with an incidence of new cases every year of 0.8%. Most of UM show a good prognosis with a 5-year survival ranging between 65–80%; however, in a quarter of patients extrauterine disease statistically worsens long-term prognosis. The pattern of metastatic diffusion of UM is different between histologies; In fact EA and PSA commonly spread through the lymphatic pathway, whereas in UL and SCC hematogenous metastases are more frequent. The lung is the most common site of extrapelvic diffusion with an incidence ranging between 2.3% and 6.1% for EC and PSA (2-4); in patients with SCC and UL pulmonary recurrence occur in 10–20% and more than 60%, respectively (5-8). Pulmonary metastasectomy for UM was successfully performed in 1930 by Torek (9) and currently this procedure is recommended in selected patients when the primary tumor is controlled or controllable with no further extrapulmonary spread, complete resection is feasible and no better therapy is available. Survival after pulmonary metastasectomy varies in the literature and is affected by several prognostic factors as number of metastases, time of presentation after primary tumor, presence of pulmonary symptoms. The aim of this study is to present our cohort of patients undergoing pulmonary metastasectomy for UM and to review the data reported in literature.
Methods
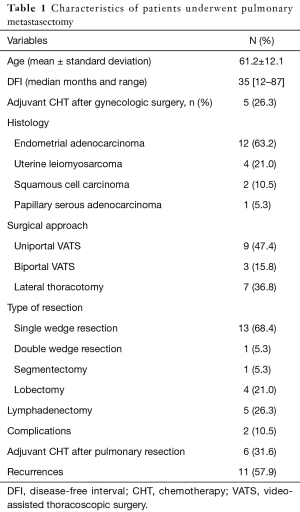
From January 1997 to December 2010 19 patients (mean age 61.2±12.1 years) underwent lung resection for metastatic uterine cancers at our Institution. Our institutional committee board approved this retrospective study. Demographic, clinical, pathological (Table 1) and survival data were retrospectively reviewed. The institutional board of our surgical department in all patients primary cancer was treated with complete surgical resection and five patients (26.3%) underwent adjuvant therapy with a platinum-based regimen. EA was the primary malignancy in 12 patients (63.2%), UL in 4 (21%), SCC in 2 (10.5%) and PSA in 1 (5.3%). All patients underwent preoperative work up including routine blood analysis, chest X-ray, cardiological evaluation with electrocardiogram and when required 2D echocardiography, pulmonary function tests and arterial blood gas analysis. Fifteen patients (79%) had serial total body computed tomography (CT) that documented the new presence of a pulmonary nodule, whereas in 4 (21%) positron emission tomography (PET) showed high uptake of a pulmonary lesion. In all patients but in 3 (16%) a solitary pulmonary nodule was present; in the others, 2 homolateral lesions were detected, in two cases in the same lobe and in 1 in different lobes. Only 1 patient (5.2%) showed symptoms as persistent cough and hemoptysis caused by a large central lesion. The disease-free interval (DFI) was calculated from the gynecologic procedure to the detection of pulmonary recurrences; the median DFI was 35 months (range, 12–87 months). In eight patients (42.1%) the DFI was less than 24 months. Survival was defined as interval from lung resection until death or last follow-up.
Full table
Overall and disease-free survival curves were calculated with the Kaplan-Meier method. Chi-squared test or Fisher’s exact test were used for univariate analysis and Cox proportional hazard regression to evaluate risk factors on survival. Variables showing a P value less/equal to 0.1 at univariate analysis were inserted in the multivariate analysis. P values less than 0.05 were considered statistically significant. All analyses were conducted with SPSS version 17.0.
Results
All the procedures were pathologically R0 resections: 14 patients (78.9%) received a wedge resection (one patient with two nodules in different lobes underwent two wedge resections), 4 a lobectomy and 1 a segmentectomy. Video-assisted thoracoscopy (uniportal or biportal) was the surgical approach in 12 wedge resections and lateral thoracotomy in others. Mini-invasive approach was preferred in case of peripheral nodule. Indication for lobectomy was the diameter of the tumor in one patient, the hilar involvement in another and the presence of two nodules in the same lobe in the other two. Sampling of hilar and mediastinal nodal stations was performed during lobectomy and segmentectomy and in 1 case positive hilar nodes were detected at histologic examination; no lymph nodes were removed in case of wedge resection. No perioperative deaths occurred and only two patients had minor complications (1 prolonged air leaks after lobectomy and 1 atelectasis requiring bronchoscopic toilette after segmentectomy). The median follow up after pulmonary metastasectomy was 54 months (range, 8–197 months). Six patients (31.6%), including the four lobectomies, the segmentectomy and the double wedge resection patients received postoperative chemotherapy based on platinum regimen. Eleven patients (58%) showed recurrences: in 8 (72.7%) the disease was disseminated (more than one site), in 3 (27.3%) was present only in the treated lung; recurrent disease was treated with chemotherapy in eight cases and with supportive care alone in 3.
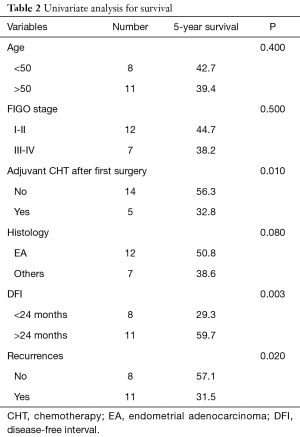
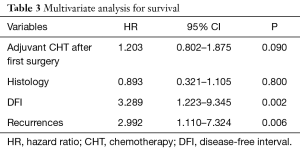
After pulmonary metastasectomy overall 5- and 10-year survival was 40.9% and 31.4%, respectively. Five-year disease-free survival was 12.8% with a median disease-free survival of 10 months. Patients treated with surgery and adjuvant therapy showed a better 5-year survival, although not achieving statistical significance (52.4% vs. 37.6%, P=0.08). Univariate analysis showed that DFI of less than 24 months (P=0.003), recurrence after pulmonary metastasectomy (P=0.02) and adjuvant therapy after initial gynecologic cancer (P=0.01) had a negative impact on survival (Table 2); at multivariate analysis only a DFI of less than 24 months and recurrence after pulmonary metastasectomy were negative factors for survival (P=0.002 and P=0.006, respectively) (Table 3).
Full table
Full table
Discussion
It is by now clear that in selected patients pulmonary metastasectomy after previous gynecologic cancers is considered the gold standard with satisfactory results in terms of long term survival. The criteria for surgical treatment are (I) good performance status of patient, (II) controlled primary tumor, (III) no evidence of extrapulmonary disease and (IV) no other better therapy available. Surgery is certainly a viable option in case of metastatic lung disease (10-12). Several experiences reported in the literature showed a 5-year survival ranging from 32.9% to 48.2% (4-7) and our data are similar with a 5-year survival of 40.9%; however, quite surprisingly, in the paper published in 2015 Adachi et al. (13) reported that the 5-year overall survival among 23 patients was 81.7% albeit 10 of these patients (43.4%) experienced recurrences and three of them died for their disease. The authors explained these results with a better selection of patients (none patient had more than four nodules) and with the R0 resection in most of the patients. Different factors have been reported to have a prognostic impact on survival: histology, age, number of nodules, presence of symptoms, length of DFI from the primary tumor, recurrence after pulmonary metastasectomy, stage of the first tumor and need of adjuvant therapy after gynecologic surgery (4,5,7,8,13,14). We have assessed several variables: short DFI (less than 24 months) and recurrence after pulmonary metastasectomy were the only independent factors affecting long-term survival. In the literature it remains controversial the real impact of DFI on survival: some authors (15,16) reported that the interval between the gynecologic cancer and onset of lung metastases was not a prognostic factor. However, other studies (4,17), similarly to our results, showed that a longer DFI was associated with improvement of survival; since the DFI cut-off varied in the different studies ranging from less/more than 12 months to less/more than 36 months, we have considered as a balanced DFI cut-off less/more than 24 months. Although also Anraku et al. (7) reported the advantages of longer DFI, they highlighted that patients with a single metastasis and a DFI shorter than 12 months showed a 5-year survival of 48.6%. Thus they recommended to not preclude pulmonary resection in these patients. Furthermore, it is not clear what is the optimal number of lung metastases to remove with curative intent; although Girard et al. (18) reported a 5-year survival rate of 28% after metastasectomy of more than eight lesions (with no significant differences on survival with patients with less than eight nodules), in most of the studies no more than four nodules were removed and patients with less metastases showed constantly a better prognosis. Only in three cases (15.7%) we removed two nodules (two lobectomies and one double wedge resection), thus we are not able to add further information.
Differently to other experiences (13,14), no patient in our series with recurrence after pulmonary resection underwent surgery again; they received chemotherapy or palliative care. Almost one third of our patients underwent adjuvant therapy with a platinum regimen after pulmonary metastasectomy showing a better 5-year survival rate respect to other patients. Five out of six of these patients (83.3%) received an anatomical resection (four lobectomies and one segmentectomy) and complete lymphadenectomy; this point could encourage a more aggressive in very selected patients treatment to improve the results, even if in our series the number of patients is limited and the differences in terms of survival are not statistically significant.
In conclusion, our results confirm that pulmonary metastasectomy after gynecologic cancers is a safe and feasible procedure contributing to achieve long-term survival when the inclusion criteria are respected. In very selected patients a more aggressive approach with anatomical resection and adjuvant chemotherapy could allow better prognosis. Since just a few series are reported in the literature, it should be preferred to plan multicenter studies with tight inclusion criteria to enlarge the numbers and obtain more validated results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our institutional committee board approved this retrospective study.
References
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review 1975-2014, National Cancer Institute. Available online: https://seer.cancer.gov/csr/1975_2014/
- Ballon SC, Berman ML, Donaldson RC, et al. Pulmonary metastases of endometrial carcinoma. Gynecol Oncol 1979;7:56-65. [Crossref] [PubMed]
- Paik ES, Yoon A, Lee YY, et al. Pulmonary metastasectomy in uterine malignancy: outcomes and prognostic factors. J Gynecol Oncol 2015;26:270-6. [Crossref] [PubMed]
- Anderson TM, McMahon JJ, Nwogu CE, et al. Pulmonary resection in metastatic uterine and cervical malignancies. Gynecol Oncol 2001;83:472-6. [Crossref] [PubMed]
- Yamamoto K, Yoshikawa H, Shiromizu K, et al. Pulmonary metastasectomy for uterine cervical cancer: a multivariate analysis. Ann Thorac Surg 2004;77:1179-82. [Crossref] [PubMed]
- van Nagell JR Jr, Rayburn W, Donaldson ES, et al. Therapeutic implications of patterns of recurrence in cancer of the uterine cervix. Cancer 1979;44:2354-61. [Crossref] [PubMed]
- Anraku M, Yokoi K, Nakagawa K, et al. Pulmonary metastases from uterine malignancies: results of surgical resection in 133 patients. J Thorac Cardiovasc Surg 2004;127:1107-12. [Crossref] [PubMed]
- Tirumani SH, Deaver P, Shinagare AB, et al. Metastatic pattern of uterine leiomyosarcoma: retrospective analysis of the predictors and outcome in 113 patients. J Gynecol Oncol 2014;25:306-12. [Crossref] [PubMed]
- Torek F. Removal of metastatic carcinoma of the lung and mediastinum: suggestions as to technique. Arch Surg 1930;21:1416-24. [Crossref]
- Internullo E, Cassivi SD, Van Raemdonck D, et al. Pulmonary metastasectomy: a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol 2008;3:1257-66. [Crossref] [PubMed]
- Lin JC, Wiechmann RJ, Szwerc MF, et al. Diagnostic and therapeutic video-assisted thoracic surgery resection of pulmonary metastases. Surgery 1999;126:636-41; discussion 641-2. [Crossref] [PubMed]
- De Giacomo T, Rendina EA, Venuta F, et al. Thoracoscopic resection of solitary lung metastases from colorectal cancer is a viable therapeutic option. Chest 1999;115:1441-3. [Crossref] [PubMed]
- Adachi M, Mizuno M, Mitsui H, et al. The prognostic impact of pulmonary metastasectomy in recurrent gynecologic cancers: a retrospective single-institution study. Nagoya J Med Sci 2015;77:363-72. [PubMed]
- Clavero JM, Deschamps C, Cassivi SD, et al. Gynecologic cancers: factors affecting survival after pulmonary metastasectomy. Ann Thorac Surg 2006;81:2004-7. [Crossref] [PubMed]
- Barter JF, Soong SJ, Hatch KD, et al. Diagnosis and treatment of pulmonary metastases from cervical carcinoma. Gynecol Oncol 1990;38:347-51. [Crossref] [PubMed]
- Seki M, Nakagawa K, Tsuchiya S, et al. Surgical treatment of pulmonary metastases from uterine cervical cancer. Operation method by lung tumor size. J Thorac Cardiovasc Surg 1992;104:876-81. [PubMed]
- Fuller AF Jr, Scannell JG, Wilkins EW Jr. Pulmonary resection for metastases from gynecologic cancers: Massachusetts General Hospital experience, 1943-1982. Gynecol Oncol 1985;22:174-80. [Crossref] [PubMed]
- Girard P, Spaggiari L, Baldeyrou P, et al. Should the number of pulmonary metastases influence the surgical decision? Eur J Cardiothorac Surg 1997;12:385-91; discussion 392. [Crossref] [PubMed]


